Authors / metadata
DOI: 10.36205/trocar5.2024015
Abstract
In this study, our objective is to report the case of intestinal obstruction secondary to endometriosis in a 45-year-old patient, who presented with a clinical history of chronic evolution of abdominal distension, constipation and pelvic pain.
Pelvic magnetic resonance imaging ( MRI) reported an extensive lesion in the anterior wall of the rectum measuring approximately 80 mm and located 9 cm from the anal verge, compromising 40 to 50% of the circumference, infiltrating up to the submucosa, compatible with deep endometriosis. Colonoscopy identified a stenosing lesion with an infiltrating appearance in the distal sigmoid, with biopsy reporting absence of endometriosis.
The approach was initially a minilaparotomy to first manage the bowel obstruction and reduce the abdominal volume. Then a laparoscopic approach was performed for segmental intestinal resection and anastomosis. The patient was discharged on the first postoperative day with full surgical recovery, with favorable evolution.
Study Objective: To describe the laparoscopic technique of segmental resection for the repair of intestinal obstruction due to deep endometriosis.
Design: Case report
Setting: Private Hospital in Curitiba, Brazil
Introduction
Endometriosis is currently one of the most prevalent and studied diseases in gynecology and is defined as the presence of endometrial glandular or stromal cells outside of the uterine cavity. It affects between 10% and 15% of women of reproductive age. In the deep infiltrating forms of the disease, the endometriosis penetrates below the surface of the peritoneum invading more than 5 mm in depth (1). Intestinal involvement is defined by infiltration of the muscularis and is estimated to occur in approximately 10% of endometriosis cases, of which 90% affect the rectum and/or the sigmoid. Other segments are less frequently affected, such as the appendix in 2.8%, ileum and cecum in 7% of the cases and the jejunum and small intestine in 3% of the cases (2). In severe cases, lumen stenosis caused by mass formation can lead to intestinal obstruction, which only accounts for 0.1% _ 0.7% and hematochezia is rare because lesions rarely invade the mucosa (3). Rectal and sigmoid endometriosis are associated with severe progressive symptoms, such as abdominal and pelvic pain, diarrhea, constipation, rectal bleeding and very rarely, to bowel occlusion (4).
Case Report
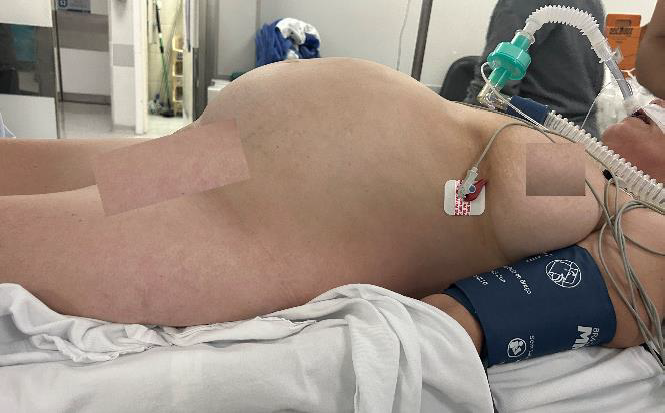
45-year-old G2/P2 woman with a clinical history of a chronic evolution of abdominal distension, constipation and pelvic pain. Surgical history includes bariatric reduction gastroplasty, cholecystectomy and total hysterectomy due to adenomyosis. On physical examination, she presented with abdominal distension, tympanic abdomen with accelerated intestinal sounds and no palpable masses were found. (Fig.1)
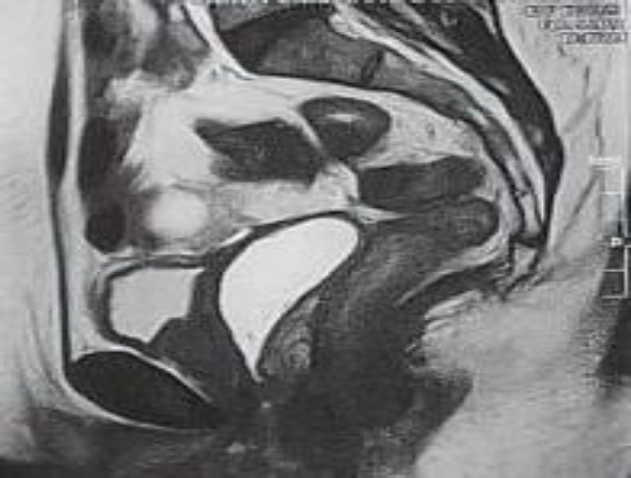
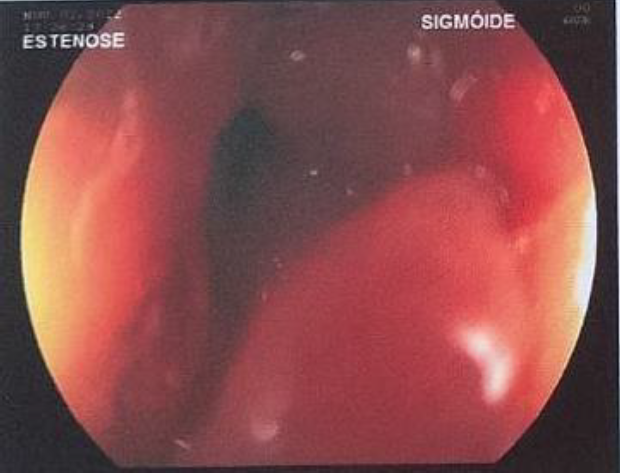
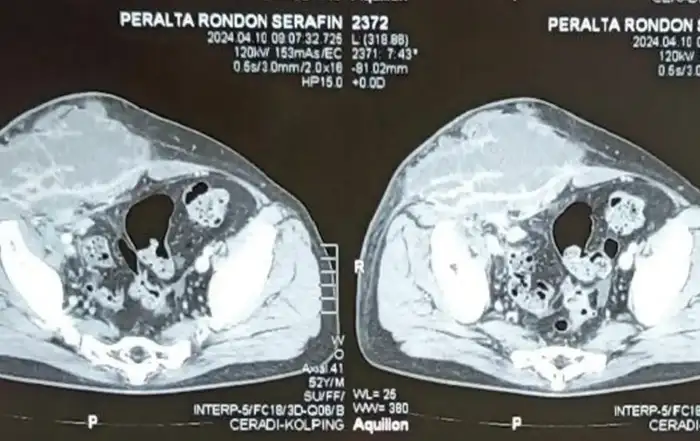
Laboratory tests were carried out with a finding of serum cancer antigen (CA) 125 concentration of 68.4 UI/ml, and the rest of the exams within normal parameters. Pelvic MRI showed an absent uterus, right ovary with a 33x36x27 mm and a 16x17x7mm hemorrhagic cysts, left ovary with a 45x39x30 mm hemorrhagic cysts, and a 15x11x11 mm and a 10x5x4 mm endometriomas. Further an extensive lesion in the anterior wall of the rectum about 9 cm from anal verge with 80 mm of intestinal extension, involving 40 to 50% of intestinal circumference, infiltrating up to submucosa compatible with deep endometriosis was described. (Fig.2) At colonoscopy, a stenosing lesion with an infiltrative appearance was identified in the distal sigmoid, with biopsy reporting absence of endometriosis. (Fig.3)
Interventions
The patient was placed in supine position and the surgery was carried out under general anesthesia. Initially, a minilaparotomy was performed with a 5 cm suprapubic incision, umlaut in planes, the sigmoid loop was identified, making a cut to introduce the 14 Fr fouchet tube (gastric drainage tube). Electrical aspiration was performed to first manage the obstruction and reduce the abdominal volume. Intestinal decompression was performed with significant improvement.
In the second stage, laparoscopy was started, with the patient placed in the lithotomy position with the arms along the body and the lower limbs abducted. Pneumoperitoneum was achieved by a Veress needle placed in the umbilicus and laparoscopic trocars were placed according to the French technique in the following way: one 10 mm trocar in the umbilicus for the zero-degree laparoscope and three 5 mm trocars under direct vision one in the right fossa slightly superior of the line between the spinae iliacae , one in the midline between the umbilicus and symphysis pubis approximately 8 cm below the umbilical trocar, and one in the left fossa slightly superior of the line between the spinae iliacae.
Gynecological Time
Step 1: Cavity inspection.
Absent uterus left ovary adhered to intestinal lesion and distended intestinal loops are observed.
Step 2: Identification of endometriotic lesions.
Endometriosis implants are identified in the anterior vaginal fornix, ovarian fossae, rectovaginal septum and rectum. The rectal endometriotic nodule measures approximately 6 cm with significant retraction of the mesocolon and extending to the lateral pelvic wall near the hypogastric nerves.
Step 3: Ureteral Identification.
The ureters were identified bilaterally and dissected with ultrasound energy using an harmonic scalpel along their entire course from the iliac vessels, ovarian fossa, uterosacral ligament, and pararectal spaces, preserving the hypogastric nerves.
Step 4: Resection of endometriotic lesions.
Resection starts from the posterior vaginal fornix, the endometriotic lesion was separated from the retrocervical and rectovaginal area using the reverse Rendezvous technique, leaving the disease adherend to the anterior face of the rectum at this time. Removal of the compromised vaginal stump and suture of the vaginal vault with poliglecaprone n°0 for continuous stitches and polydioxanone n°0 for separated stitches.
Bowel Time
Step 5: Approach to the pararectal spaces.
Using the ureter as anatomical mark, the medial and lateral pararectal spaces are dissected. As the hypogastric nerve is well identified, the dissection of hypogastric branches follows until its confluence with the branches of the sacral roots. The dissection is performed bilaterally.
Step 6: Rectosigmoidectomy.
The vascular and nerve sparing technique is elected to achieve a proper mesentery dissection, as the rectosigmoid segment is prepared and detached for the resection of the segment compromised by the endometriosis nodule.
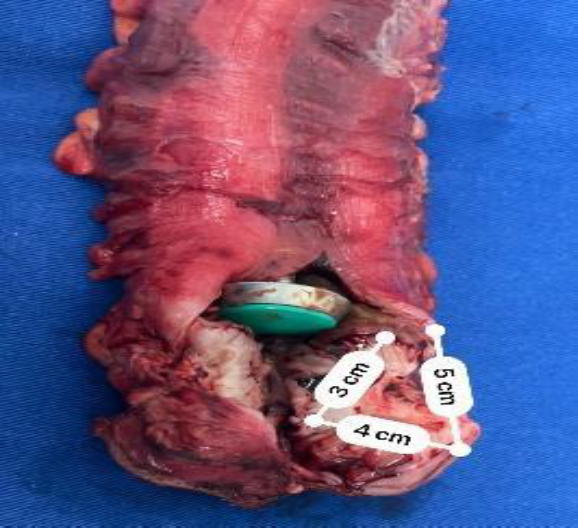
A 12 mm suprapubic trocar is placed to introduce a 45 mm linear endostapler for bowel resection, which is achieved with two charges of stapling, developing the rectal stump. (Fig.4)
Step 7: Protected colon exteriorization.
By the suprapubic incision the colon is exteriorized and the lateral wall of the proximal stump is opened for the placement of the 33 mm circular endostapler for a latero-terminal anastomosis. The segmental resection and the stump closure is carried out using the linear stapler. It was decided to use a latero-terminal anastomosis because the sigmoid diameter was around 10 cm due to the obstruction and three charges of 45mm stapler were necessary to complete the section and to close the sigmoid. The staple line was reinforced with polydioxanone 3-0 seromuscular suture and the suprapubic incision is closed. The stump with the anvil is pushed back into the abdomen.
Step 8: Latero-terminal colorectal anastomosis.
The circular stapler is inserted trans anally and its trocar perforates the rectum stump and is attached to the anvil placed in the sigmoid stump, achieving a tension-free latero-terminal anastomosis. A staple line reinforcement is made with 3-0 polydioxanone cross mattress suture at each lateral angle of the anastomosis. At the end, the patency of the anastomosis is tested by insufflating the rectal stump with air, without air leak sign. due to the extreme dilatation of the sigmoid and the high risk of fistulas in this situation a tubulo-laminar drain was placed in the pelvis and exteriorized at the right iliac fossa. However, it is important to emphasize that the team usually does not use drains in colorectal resection for endometriosis. (Fig.5)
Measurements and main results
The interval between minilaparotomy and the start of laparoscopy was 30 min.
The procedure lasted 180 minutes and the estimated blood loss was 100 ml. During hospitalization she remained with a Foley catheter. The patient followed an exclusively liquid diet for six hours after the procedure. The patient had an uneventful intraoperative and postoperative course and was discharged on the first postoperative day with tubulo-laminar drainage for seven days. She was able to resume work 15 days after surgery. At her 4-week follow-up, she reported resolution of her symptoms. Pathological examination of the specimen revealed a large intestine 18.5 cm long and 10 cm in diameter, with an endometriotic lesion surrounding the colorectal wall (pericolic, perirectal to submucosal), with lesion-free proximal and distal bowel resection margins.
Discussion
Patients with obstructive symptoms from intestinal deep endometriosis should undergo surgical resection. When endometriotic lesions involve the rectum and/or sigmoid colon beyond the internal muscular layer and more than 40% of the circumference the recommendation is segmented intestinal resection (5). It has been described in the literature that the construction of an anastomosis under tension or with poor blood supply increases the risk of anastomosis leakage that can cause postoperative infection and sepsis. However, for terminal- terminal, colo colic anastomosis with functional staples this view has been questioned by scrutiny.
A diverting shield ostomy does not prevent leakage as such, but it should decrease the life-threatening complications of an anastomotic leak (6). When comparing laparoscopic colonic resection with laparotomy in patients with intestinal deep endometriosis, both options lead to similar symptomatic improvement after surgery, but those who underwent laparoscopic treatment had significantly lower rates of surgical complications (7). As of February 2021, there have been 11 reported in PubMed cases of acute colonic obstruction due to endometriosis are described with nine Hartmann’s procedure or direct anastomosis with defunctioning stoma were performed, open or laparoscopic (8). Two cases of stent placement in an intestinal colonic obstruction caused by endometriosis, as a bridge to laparoscopy surgery with successful results have been reported (9)
The reported clinical case shows that the chronic evolution of the pathology caused an important increase in the diameter of the small and large intestine in the rectosigmoid region. The patient did not present total intestinal obstruction; however, the abdomen was highly distended.
Unlike most case reports colonic obstruction due to endometriosis resolved Hartmann’s procedure or direct anastomosis with defunctioning stoma, our approach was minimally invasive, with successful results.
Conclusion
Minimally invasive approach to intestinal obstruction due to intestinal deep endometriosis is feasible if the dilated colon can be decompressed before entering the abdominal cavity.
Video
References
Fig.1: Patient in operating room with significant abdominal distention
Fig.2: Pelvic magnetic resonance imaging with extensive lesion in the anterior wall.
Fig.3: Colonoscopy showing the infiltrative endometriotic lesion in the distal sigmoid.
Fig.4: Large intestine bowel resection piece.
Fig.5: Tubulo-laminar drainage at the right iliac fossa.