Authors / metadata
DOI: 10.36205/trocar5.20240010
Abstract
Introduction: Endometriosis after menopause, which affects 2-5% of women, is difficult to diagnose and treat, largely due to the lack of non-invasive diagnostic tools, absence of classic menstrual symptoms, and awareness1. It is often diagnosed in women of reproductive age with characteristic symptoms of dysmenorrhea, menorrhagia, and infertility. The absence of these symptoms poses unique diagnostic difficulties when encountered in postmenopausal women. Deep endometriosis (DE) describes the invasion of lesions exceeding 5 mm. Delayed surgical intervention of DE of the urinary tract may result in devastating consequences, including hydronephrosis and kidney loss.
Case: A 61-year-old G2P2 postmenopausal woman presented to the emergency department for acute left lower quadrant and flank pain. The patient had a history of dyspareunia, endometriosis, and use of vaginal estrogen cream. Abdominal imaging showed a 4.2 x 3 cm pelvic mass, left hydronephrosis, and ipsilateral loss of kidney function. Intraoperative findings included DE from the left pelvic sidewall to the space of Yabuki encompassing the distal left ureter. The patient underwent a total laparoscopic hysterectomy, bilateral salpingo-oophorectomy, excision of endometriosis with ligation and excision of the distal ureteral mass, cystoscopy, and laparoscopic left nephroureterectomy with the Minimally Invasive Gynecologic Surgery service and Urology.
Conclusion: The absence of characteristic symptoms of endometriosis increases the risk of delayed diagnosis and surgical intervention in postmenopausal women. Our case displays how DE can present in a non-traditional population, identifies exogenous estrogen use as a potential risk factor, highlights the surgical importance of the Yabuki space, and addresses gaps in Sampson’s theory of pathogenesis.
Introduction
Endometriosis is a prevalent condition affecting approximately 10% of reproductive age women2. It is defined by ectopic endometrial-like tissue outside the uterus3. Yet, the challenge posed by this condition is not confined to this demographic. Notably, an estimated 2-5% of postmenopausal women are affected by endometriosis, often presenting without the typical symptoms of dysmenorrhea or infertility characteristic of endometriosis in reproductive-aged women4. In postmenopausal women, endometriosis may present with nonspecific pelvic pain, dyspareunia, and symptoms related to the location of the lesion1. While involvement of the genitourinary (GU) tract constitutes about 1% of all endometriosis cases, it is seen in up to 53% of cases of deep endometriosis (DE)5,6. A predilection for bladder endometriosis exists in GU endometriosis with a prevalence ratio of bladder-to-ureteral-to-kidney involvement of 40:5:15. Alarmingly, the majority (65%) of urinary tract endometriosis cases present without classic urinary symptoms, with misdiagnosis leading to deleterious terminal kidney loss in severe cases7. Symptomatic patients may report flank pain, pelvic pain, and rarely gross hematuria8,9. Postmenopausal symptomatic endometriosis is often seen in women with a history of gynecologic surgery for endometriosis, most commonly hysterectomy, and with the use of hormone replacement therapy5, 9-11. Herein, we present a case of DE in a postmenopausal woman with unilateral kidney failure to address how endometriosis can present in an uncommon demographic, highlight the surgical importance of the Yabuki space, and address gaps in Sampson’s theory of endometriosis pathogenesis.
Case report
A 61-year-old G2P2 postmenopausal woman presented to the emergency department (ED) for acute left lower quadrant and left flank pain rated 10/10 that suddenly woke her up from sleep. The patient reported a longstanding history of dyspareunia and abdominal wall endometriosis. Surgical history was significant for 2 prior cesarean sections and 2 cesarean scar abdominal wall endometrioma excisions in 1996 and 1998 at an outside hospital. She was of normal weight (BMI 24.38) and was using vaginal estrogen cream for genitourinary symptoms of menopause at the time of presentation. Her last menstrual period was in 2010 after an endometrial ablation and a treatment course of Leuprolide injection for abnormal uterine bleeding. Three days prior, she presented to an outside hospital for abdominal pain and computed tomography (CT) scan of the abdomen showed an enlarged left ovary (3.9 x 2.3 cm) and subacute left hydronephrosis from a downstream ureteral obstruction.
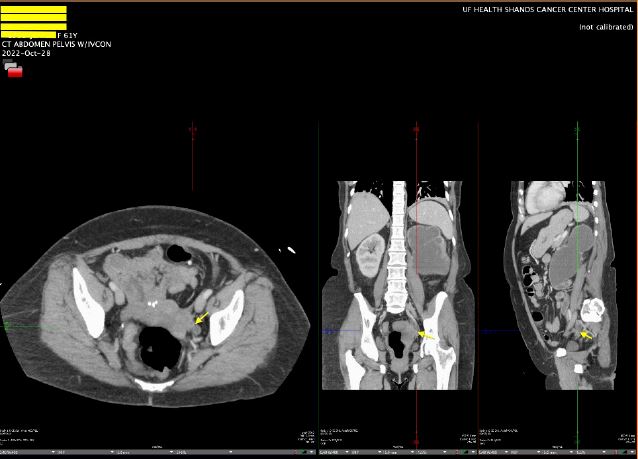
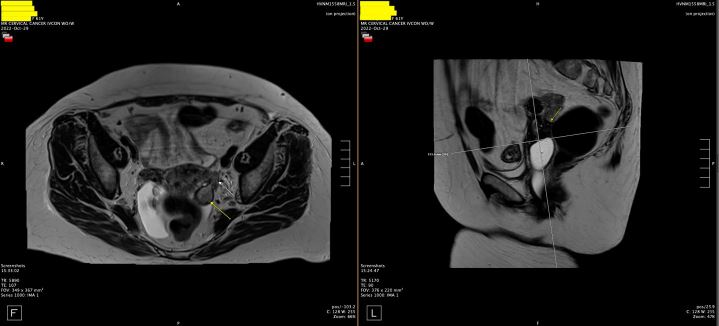
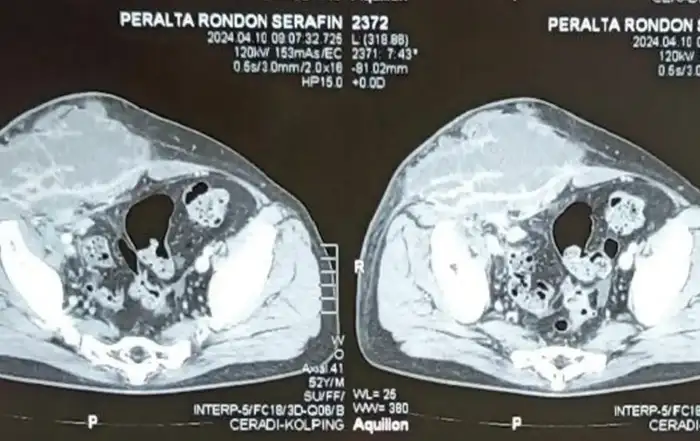
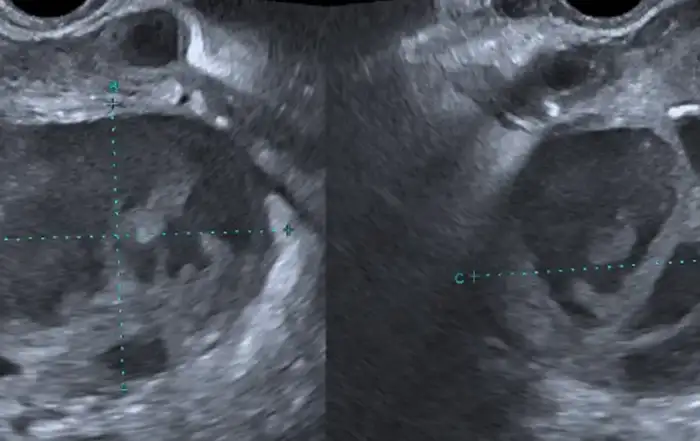
The patient was discharged with an outpatient Urology appointment. In our ED on presentation, the initial radiology read on CT scan of the abdomen was notable for a mass along the left distal cervix measuring 4.2 x 3 cm concerning for cervical cancer and severe left hydroureter with a transition point just above the left ureterovesical junction, chronic left hydronephrosis, and left renal cortical thinning consistent with a chronic process (Figure 1). The gynecology service was consulted to evaluate for possible cervical cancer. A pelvic exam was notable for significant pelvic floor tenderness, with a normal-appearing cervix, normal-sized non-tender uterus, and a Papanicolaou smear was collected. Urology was consulted, and the patient underwent a percutaneous left nephrostomy tube placement. After the nephrostomy tube placement, repeat imaging via pelvic magnetic resonance imaging (MRI) revealed a normal cervix, a linear area of fibrotic scarring extending from left cervix to the ventral aspect of rectal vault, thickening of the left round ligament, and tenting of the left ovary by a spiculated left pelvic lesion involving the left ureter, concerning for deep endometriosis (Figure 2). Left kidney function was found to be 28% on renal lasix.
This was considered an overestimation of a nonfunctional left kidney supported by cortical atrophy on CT scan and scant output from nephrostomy tube. Patient preference was for nephrectomy at the time of GYN surgery. The patient’s vaginal estrogen was discontinued, and she was started on anastrozole with a plan for a total laparoscopic hysterectomy, bilateral salpingo-oophorectomy, endometriosis resection, cystoscopy, and left nephro-ureterectomy with the Minimally Invasive Gynecologic Surgery (MIGS) and Urology service.
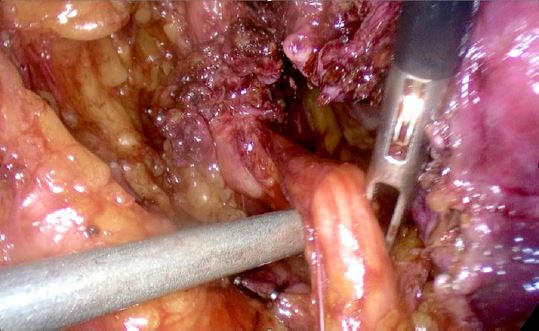
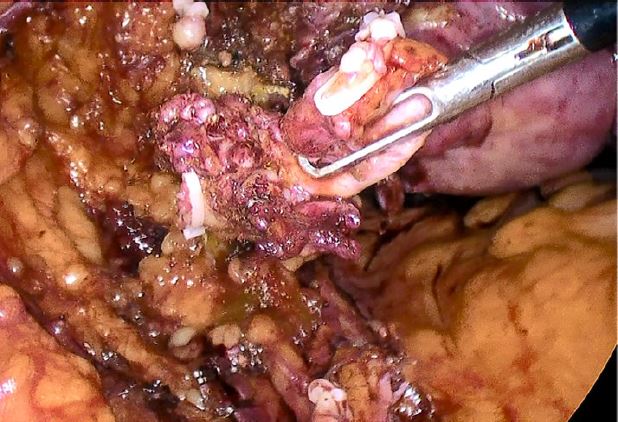
The patient was taken to the operating room and intraoperative findings were notable for a normal-sized uterus with multiple small intramural myomas, dense adhesions of the bladder to the lower uterine segment, left ovary and fallopian tube with dense adhesions to the pelvic sidewall, and a 3 cm deep endometriotic nodule in the left parametrium. The nodule extended from the left pelvic sidewall to the retroperitoneal space of Yabuki, bordered by the anterior surface of the uterus posteriorly and the ureter inserting into the bladder12, encompassing the left distal ureter (Figures 3, 4, 5).
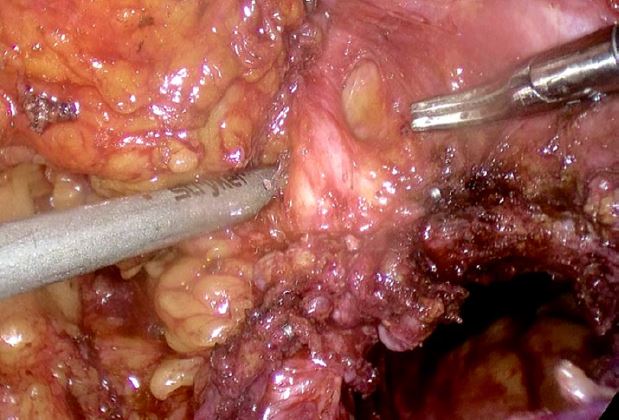
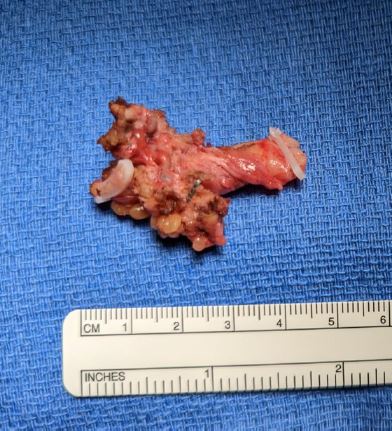
Cystoscopy was only notable for an absent left ureteral jet. The patient underwent a total laparoscopic hysterectomy with bilateral uterine artery ligation at the origin, bilateral salpingo-oophorectomy, excision of endometriosis with ligation and excision of the distal ureteral mass (Figure 6), and cystoscopy by the MIGS service. Urology performed a laparoscopic left nephro-ureterectomy. There were no intraoperative or postoperative complications. The estimated blood loss was 100 ml. The patient was admitted for 2 days for routine postoperative care. At 4 weeks postoperatively, the patient was doing well and had resolution of her symptoms.
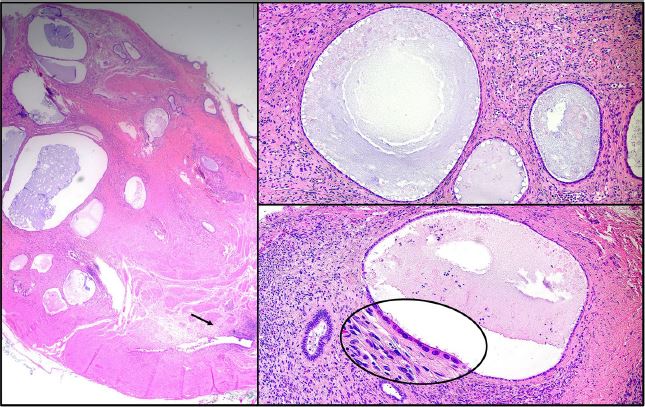
The pathology of the left ureter showed extensive extrinsic polypoid endometriosis composed of an admixture of endometriotic stroma and atrophic glands showing cystic changes with features resembling those of an endometrial polyp (Figure 7). In addition, there was adenomyosis of the uterus, seromucinous adenofibromas of the ovaries with foci reminiscent of endometriosis, and para-tubal endometriosis of the right fallopian tube.
Discussion
DE presenting as hydronephrosis in a postmenopausal woman is a rare finding. To date, there have been twelve reported cases of ureteral endometriosis in postmenopausal women8-10,13-15 . This is likely an underestimation, as the literature commonly omits information on menopausal status and is more likely to report the age of the patient population. Prompt diagnosis of DE and surgical management are necessary to reliably exclude malignancy, prevent loss of kidney function, and to improve the quality of life4. It is estimated that 30% of patients with ureteral endometriosis have experienced reduced kidney function at the time of diagnosis16. According to the group of Kvaskoff et al. endometriosis carries a risk of malignant transformation with a summary relative risk of ovarian cancer of 1.9317. Medical management of refractory symptoms in pre-menopausal patients includes combination oral contraceptives, progestins, levonorgestrel intrauterine system (LNG-IUS), GnRH analog, and aromatase inhibitors3. While there is limited data regarding the management of postmenopausal endometriosis, aromatase inhibitors have been shown to improve symptoms refractory to surgical excision in case reports18. Endometriosis is most commonly diagnosed in women of reproductive age partially due to the key role estrogen plays in its pathogenesis. Some studies have identified hormone replacement therapy (HRT) with estrogen-only as a risk factor for progression and malignant transformation of endometriosis3. As a result, it is advised to use combination hormone replacement therapy (HRT) when indicated regardless of the presence of a uterus in individuals with endometriosis3. However, the heterogeneity of endometriosis in different contexts makes it difficult to prove a single etiopathologic model for this complex disease. In this case, we postulate that the direct vaginal estrogen exposure of our patient may have contributed to the progression of a pre-existing endometriotic lesion involving the ureter, leading to polypoid endometriosis which was not seen in the endometriotic lesions at other sites from the same patient. Polypoid endometriosis refers to extrauterine endometriotic tissue with histologic features reminiscent of an endometrial polyp, which can clinically mimic a malignant tumor. Although endometriosis typically affects younger women with <5% of endometriosis cases seen in postmenopausal women, polypoid endometriosis more frequently occurs in the latter population. In the largest series, 60% of polypoid endometriosis cases were detected in patients over 50 years of age and it has been speculated that hyperestrogenism, particularly due to exogenous hormones, was “almost certainly” a contributing factor in some of the cases.19 Further studies are needed to assess the effect of exogenous estrogen on endometriosis through both oral and topical HRT. Recommendations suggest the use of progestins in addition to estrogen hormone replacement to treat vasomotor symptoms of menopause in women with endometriosis who require HRT4,20.
Our case further highlights the clinical importance of the anatomical knowledge of Yabuki space. Endometriosis of the anterior vaginal wall may impact the ureter where it crosses the vagina lateral from posterior to anterior. This finding increases the significance of this potential space in women requiring surgical removal of ureteral endometriosis. Furthermore, we highlight the low diagnostic accuracy of imaging in nonspecialized centers despite the high operator-dependent specificity and sensitivity of ultrasound and MRI in high-volume endometriosis centers around the world21, 22.
The findings presented in this case highlight the need for robust research into noninvasive diagnostic tools and alternative theories of endometriosis pathophysiology beyond Sampson’s theory that endometriosis is initiated or exacerbated by retrograde menstruation2. Postmenopausal women are at risk for endometriosis despite having ceased menstruation. Although this patient had a history of endometriosis in her reproductive years, it appears that she developed new lesions and/or they progressed during menopause. This conclusion is supported by findings of extensive endometriosis and related lesions at multiple sites with an acute onset of symptoms.
Alternative theories that mandate further clinical research include the genetic-epigenetic theory, the mulleriosis theory, and the role of the reproductive and gut microbiome and its metabolome on endometriosis. The genetic-epigenetic theory posits that endometrial lesions are the result of genetic and epigenetic incidents accumulated over time and that typical, cystic, and deep endometriosis lesions are uniquely influenced by a variety of environmental factors23. Our patient’s extensive history of abdominal surgery indicates increased inflammation and stress that could have advanced an existing predisposition to endometriosis. The mulleriosis theory suggests that deep endometrial lesions are the product of previously undifferentiated cells of the mullerian duct that develop into endometrial-like cells in response to estrogen and other factors24. The presence of deep endometriosis with the use of local estrogen in our patient bolsters this theory. The bacterial contamination theory argues that gastrointestinal and genitourinary microbial dysbiosis produces a cycle of worsening inflammation, adhesion, and angiogenesis that induces the growth of endometriotic lesions25,26. Lower estrogen levels in menopause result in vaginal microbiome perturbations27. Therefore, microbiome dysbiosis may have a role in endometriosis in this population.
Conclusion
We present the diagnosis and treatment of a postmenopausal woman with deep endometriosis of the ureter that resulted in terminal kidney loss. It is important to consider the diagnosis of endometriosis in this population as nonspecific pelvic pain may be the only early presenting feature. We highlight the use of topical estrogen as a potential risk factor in the growth of existing endometriotic lesions and the space of Yabuki as a key surgical landmark in the excision of DE. This case underscores the urgent need for research into menstruation science and endometriosis.
References
Figure 1: CT Abdomen Pelvis with left hydroureter and chronic atrophy of ipsilateral kidney
Figure 2: Pelvic MRI with fibrotic scarring from left cervix to the ventral aspect of rectal vault, thickening of the left round ligament, and tenting of the left ovary by a spiculated left pelvic lesion involving the left ureter
Figure 3: Left hydroureter with occlusive distal deep endometriosis nodule
Figure 4: Development of left distal ureter in Yabuki space
Figure 5: Left hydroureter segment with deep endometriosis
Figure 6: Excised ureteral gross lesion
Figure 7: Pathology of the left ureter showing extensive polypoid endometriosis