Authors / metadata
Abstract
Uterine myomas are very commonly found in women of reproductive age. Diagnostic and simultaneous operative hysteroscopy is the cornerstone of treatment of submucous myomas. This non-systematic review aims to outline the role of diagnostic hysteroscopy in patients with submucous myomas, and the surgical assessment of these myomas as regards technique of surgery and level of difficulty before initiating myoma resection.
Introduction
Uterine myomas are the most common benign pelvic tumours of the female genital tract [1]. Their incidence is approximately 25%–30% of all women in the reproductive age, and can be higher depending on race, family history, and genetics. Submucosal fibroids have a consistent association with heavy menstrual bleeding and infertility [2].
Hysteroscopy is the most commonly used tool for diagnosis and simultaneous treatment of patients suffering from abnormal uterine bleeding, recurrent abortions and infertility. Among many other pathologies that are responsible for these symptoms, uterine myomas are one of the most commonly found. Diagnostic hysteroscopy plays an important role in decision making regarding the treatment of submucous myomas. Hysteroscopic myomectomy is the first line minimally invasive and conservative surgical treatment. In this non systematic review, we will attempt to present the exact place of hysteroscopy in patients with submucous myomas from an exclusively diagnostic perspective.
The importance of Imaging
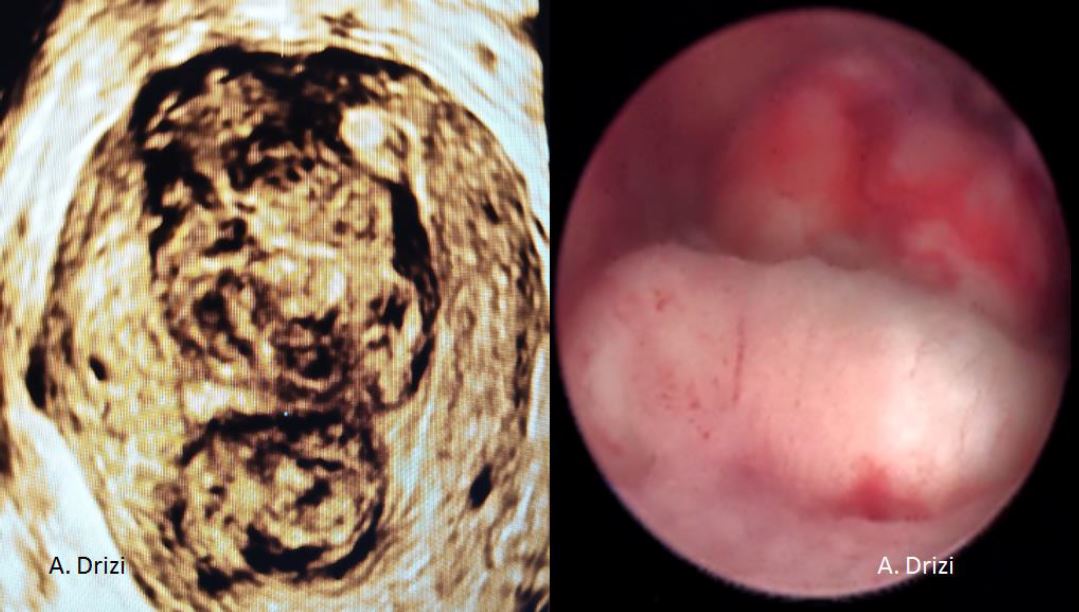
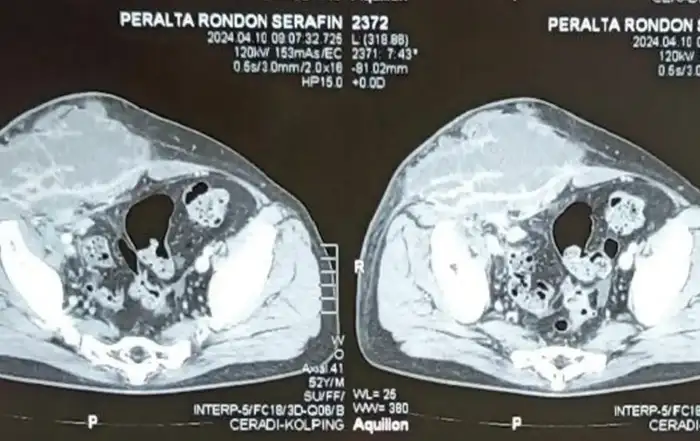
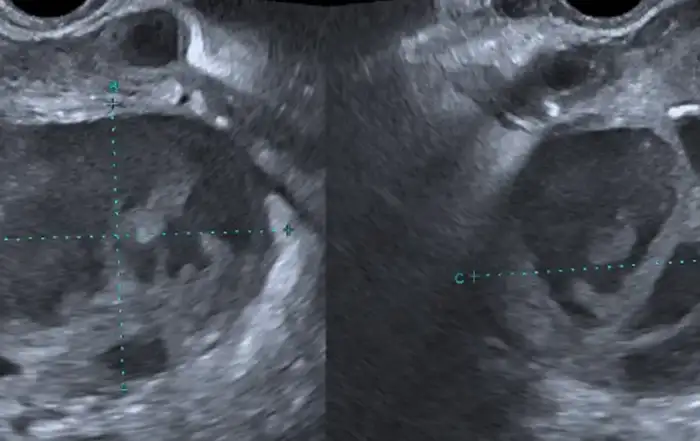
Before a surgery can be planned, a thorough assessment of the size, number and location of the fibroids is done. Conventional 2D ultrasound is sufficient to localize and map the fibroids in most cases. A saline infusion sonography delineates the cavity and provides valuable information about the extent of intra cavitary extension in case of submucous myomas. More detailed fibroid mapping using a high resolution 3D ultrasound or MRI may be used in larger or multiple fibroids, or when a diagnostic confusion arises to differentiate a fibroid from focal adenomyosis [3] [fig 1].
Many of the factors involved in assessment and planning of the surgical details are available from the imaging itself, and only need to be confirmed by diagnostic hysteroscopy before operative removal starts. A more detailed account of this is available in the article “Diagnostic hysteroscopy: patient assessment and preparation” in this issue.
Patient preparation
The patient is preferably scheduled for surgery in the early proliferative phase of the menstrual cycle. This ensures that the endometrial lining is at its minimum thickness, and smaller fibroids are not missed during the primary examination because of being buried below the thick endometrial layer.
If the timing of surgery cannot be decided in accordance with the menstrual cycle, or for patients who have irregular cycles, pre operative administration of GnRH analogues helps to thin the endometrial lining [4]. However, if the diagnostic step through hysteroscopy is necessary, especially in patients with intermenstrual abnormal uterine bleeding, hormonal treatment will represent a diagnostic bias and hence needs to be avoided. In these cases, it is best to schedule hysteroscopy in the early or mid proliferative phase.
The purpose of performing a diagnostic hysteroscopy
The primary purpose of performing a diagnostic hysteroscopy is for assessing the feasibility of the surgery, operative time, assessment of risk and the need for a concomitant diagnostic laparoscopy. It also helps to differentiate a typical submucous myoma from other intracavitary pathologies like a polyp, an adenomyoma/ atypical adenomyoma, and a cerebroid or nodular carcinoma, to name a few [fig 2-6]. A concomitant biopsy can also be performed in an office setting, if the patient is scheduled for an operative procedure under anaesthesia at a later date or if the tumour is enucleated and left inside the cavity for spontaneous expulsion.
Procedure
The patient, under anaesthesia is placed in the lithotomy position, or is comfortably seated in an examination chair specifically designed for an outpatient procedure.
The authors prefer to use a 2.9 mm 30 degree hysteroscope with a bettochi sheath for performing the procedure under or without anaesthesia. A hysteromat with a set pressure of 120 mm Hg is used, at a flow rate of 0.5 to 1 litre per minute. For outpatient procedures, a 1.9 mm telescope, when available, may allow an easier entry into the cavity, particularly in patients with stenosis of the internal os.
Intra operative assessment of the myoma(s)
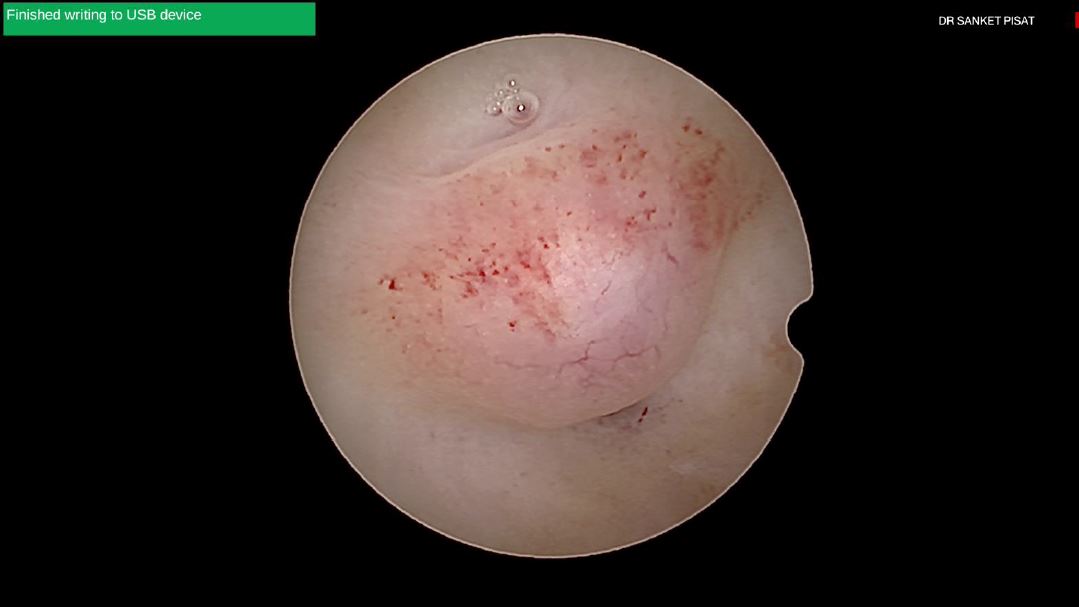
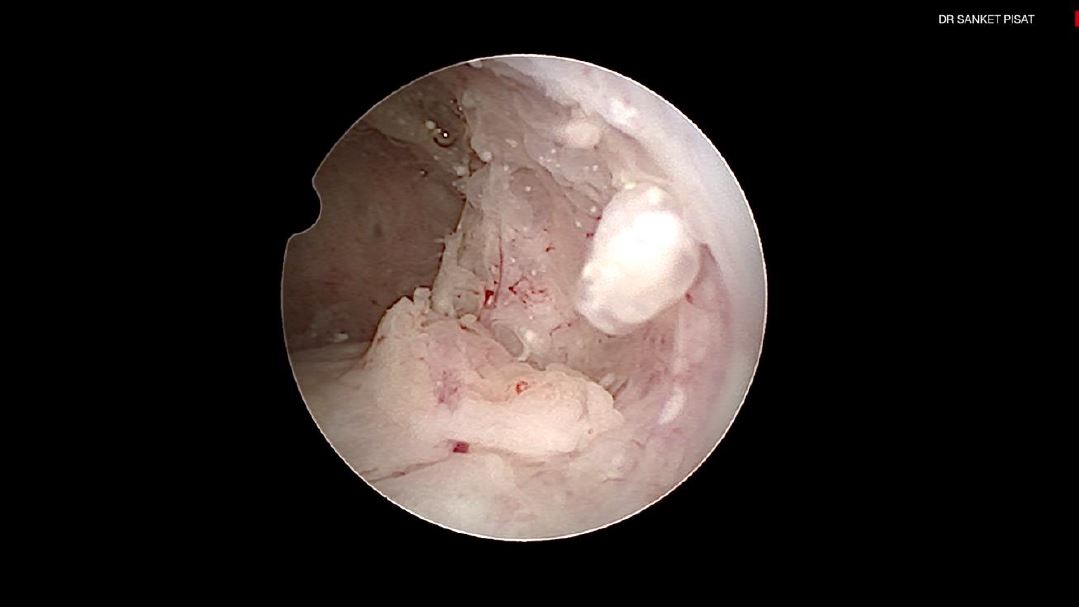
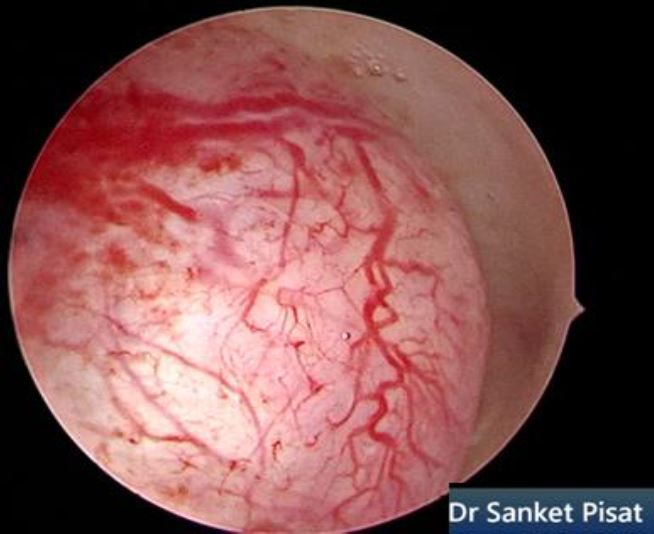
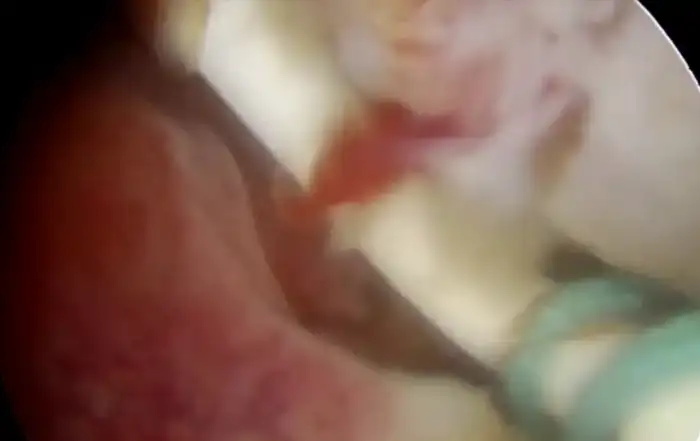
Typically, a submucous myoma is seen as an inward projection of the uterine wall. More intracavitary myomas (FIGO type 0/ type 1) are easily identified as obvious intra cavitary masses projecting from one of the walls or the fundus. Type 2 and 3 fibroids may often be hidden from view due to the overlying endometrial hypertrophy, and hence the importance of performing the procedure in the post – menstrual phase. A typical vascular architecture of fine blood vessels is seen over the surface, visibly different from the endometrium surrounding the mass [fig 2].
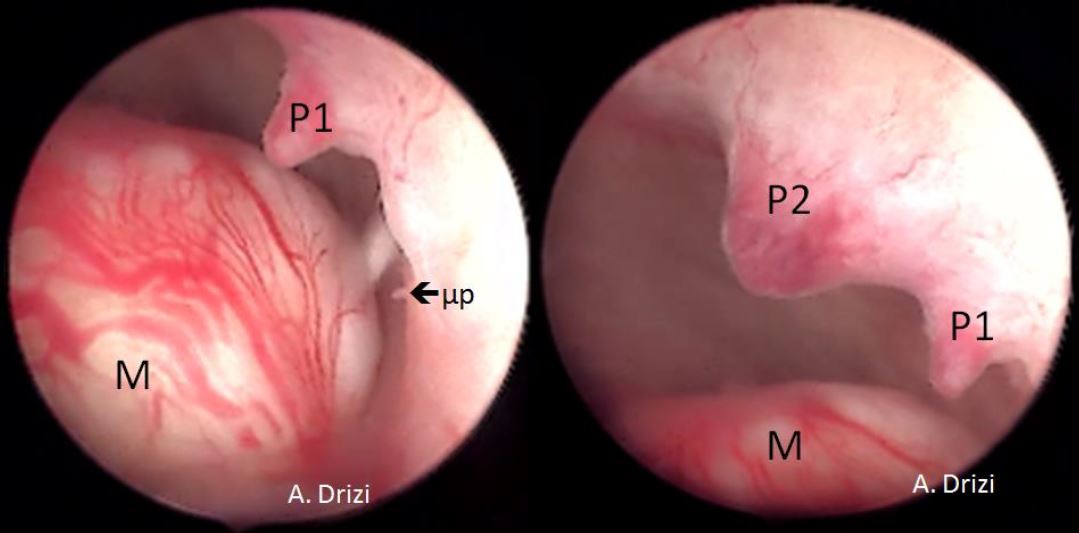
It is firstly important to differentiate a submucous myoma from a polyp. A polyp is seen as a fleshy mass that is soft in consistency, whereas a fibroid is usually harder and has a noticeably different vascular architecture over its surface [Fig 3,4].
A gentle attempt to scrape off the endometrium over the mass with the tip of the telescope can also differentiate a polyp, whose base is friable and easily disturbed, from a myoma which does not move on touching, but sheds away its covering endometrium to expose the surface more distinctly [fig 3].
Focal adenomyosis may also mimic a submucous myoma. However, some features differentiate adenomyosis from fibroids. Hysteroscopic signs of adenomyosis are: 1 Irregular endometrium with superficial openings; 2 irregular subendometrial myometrium (whorled, fibrotic, etc.); 3 absence of typical myometrial architecture during endometrial resection [5].
Atypical polypoid adenomyoma (APA) is another entity that looks like a submucous myoma, as a whitish mass protruding into the uterine cavity. It can be differentiated from a myoma only by histological examination, which shows atypical endometrial hyperplasia accompanied by nuclear enlargement and squamous metaplasia, with spindle cell hyperplasia in surrounding stromata [6].
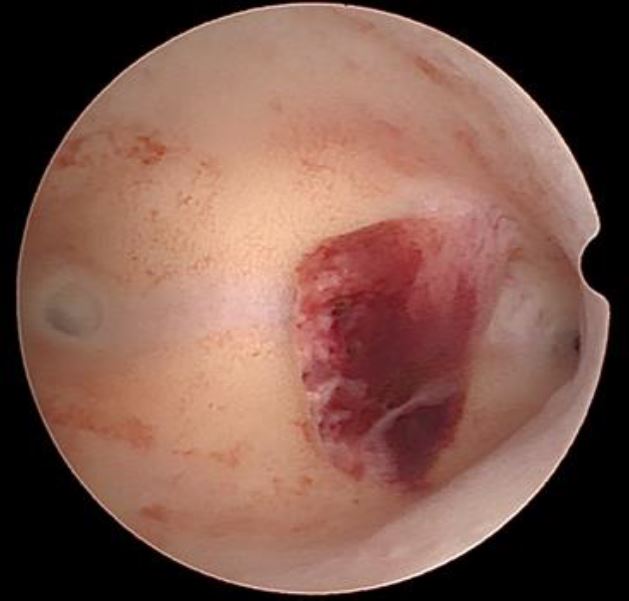
Endometroid adenocarcinoma. The visual diagnosis is generally based on the presence of a gross distortion of endometrial cavity, because of focal or extensive nodular, polypoid, papillary, or mixed patterns of neoplastic growth. Focal necrosis, friable consistency, and atypical vessels are other features almost invariably associated with endometrial cancer and easily detected by hysteroscopic inspection [fig 5].
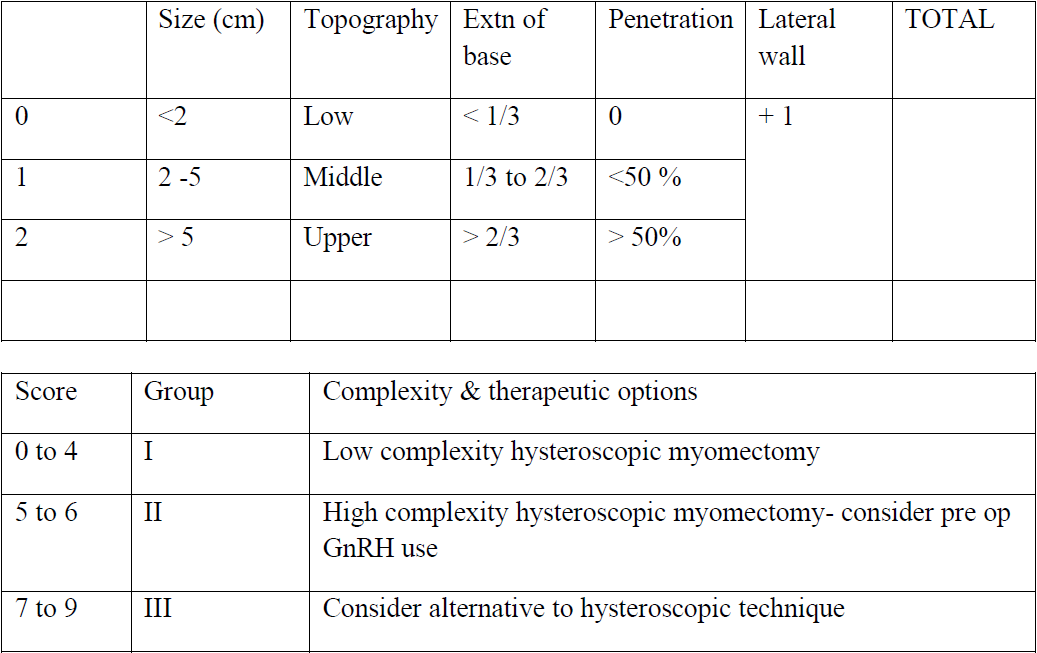
Pre operative imaging and fibroid mapping provides a very good assessment of each fibroid based on size, number, depth and location in the uterine cavity. Various classifications such as the FIGO classification, the Wamsteker classification and the STEP W classification have been used to plan the surgery. Of these, the STEP W classification is the one that closely co relates to the intra operative findings [2] [table 1].
Though these parameters are discussed independently, the final surgical decision is decided based on a cumulative assessment of all factors put together, not individually.
a. Size of the myoma(s) : hysteroscopic myomaresection is, in principle, different fromlaparoscopic myomectomy as it deals with the volume of the fibroid which has to be removed piecemeal, vs laparoscopic myomectomy where the diameter, not volume of the fibroid has to be considered for removal. Consequently, a small increase in diameter by one or two centimetres does not significantly alter the difficulty level of the surgery in laparoscopic myomectomy. However, in hysteroscopic myoma resection, the volume of the fibroid (3.14 X radius3) has to be removed. Hence, an increase in fibroid diameter by even one cm significantly increases the difficulty level and operative time.
For most surgeons, a size of 3 cm or less is an acceptable mass that can be removed without the risk of complications like fluid overload. However, this also depends on the depth of penetration of the myoma into the cavity. For fibroids that exceed 3 cm, a possibility of two stage surgery needs to be discussed with the patient before starting the case [fig 6].
b. Number of fibroids: The total number offibroids to be removed determines the timeand ease of surgery. A patient with twosubmucous myomas of 2 cm each will take asmuch time to remove as a patient having asingle 4 cm fibroid. As in the case of largefibroids, the possibility of needing a twostage surgery must be explained beforehand.
c. Location of fibroid in the uterine cavity: afibroid located lower down in the isthmus ismore easily resectable than a fibroid locatedat or closer to the fundus. Likewise, it iseasier to resect a fibroid located in theanterior and posterior wall than over thelateral wall
d.Extension of the base: a fibroid that coversone third or less of the wall is easier to resectthan a fibroid having more than one third ortwo third of extension over the wall.
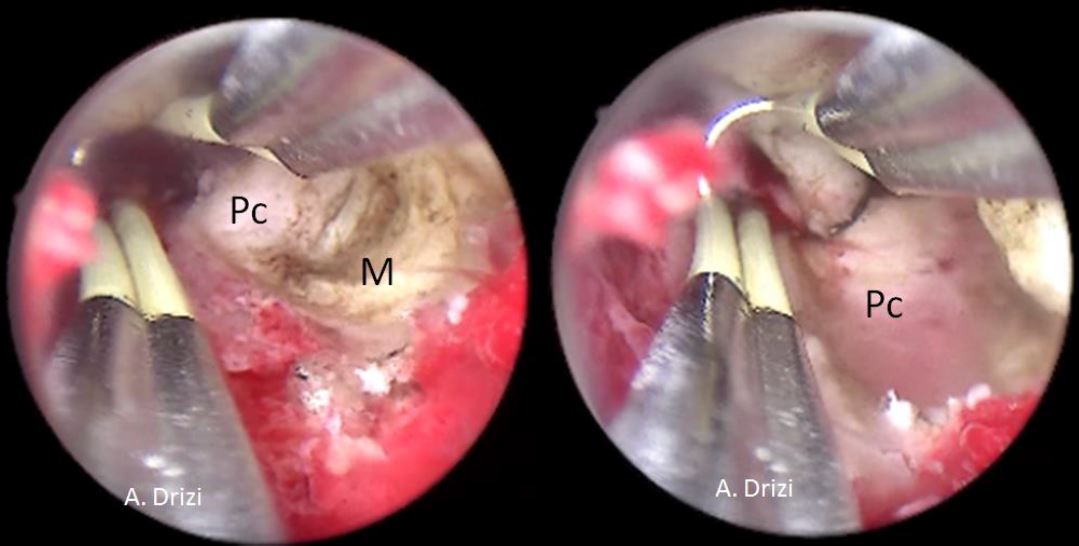
e. Depth of penetration: The depth ofpenetration of the fibroid into theendometrial cavity is assessed. A fibroid thatis more intra cavitary is easier to resect thana fibroid that is more deeply embedded intothe uterine wall. Recognition of the fibroidpseudocapsule by means of its colour andtexture, which is different from the myoma,allows the surgeon to define the end point ofsurgery [fig 7]
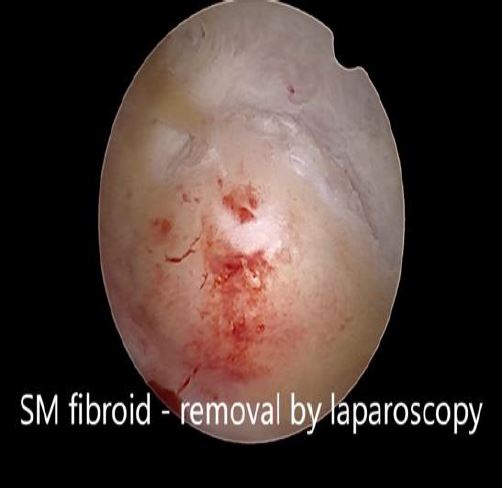
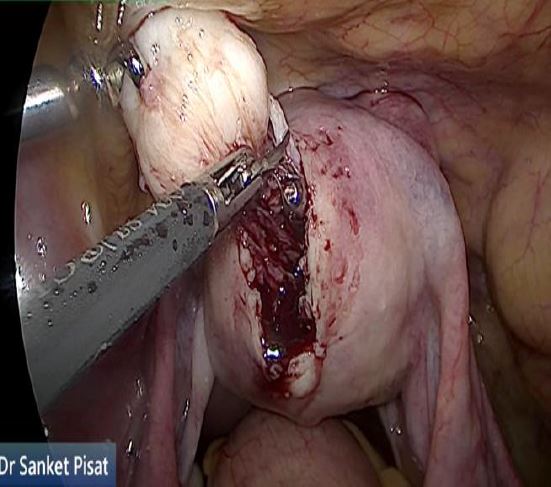
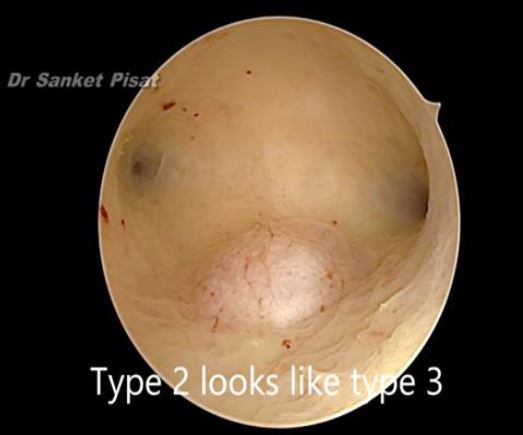
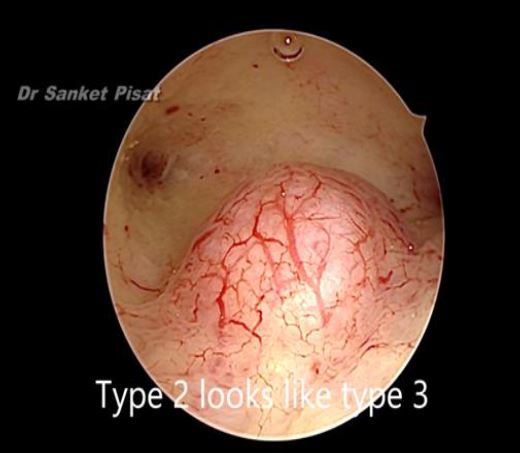
In these patients, a specific point to consider is whether the fibroid can be categorized into the type 2 to 5 category. This is a fibroid that has a bulge seen submucosally, but also extends up to the serosal margin because of its size. This fibroid requires removal by laparoscopy instead of hysteroscopy, and ensures that the entire mass can be removed in a single stage surgery, without the risk of dangerous fluid overload. Also, the endometrial lining is not subjected to extensive shaving by electrocautery, and hence post operative outcomes as regards fertility may be better [fig 8,9].
While performing the hysteroscopy, one important point to be noted is that the intra cavitary portion of the fibroid is always pushed away from the cavity by the pressure of the distending saline. Hence, while assessing the size and intra cavitary extension of the fibroid, it is vital to reduce the distension pressure to see how much the fibroid bulges into the cavity [fig 10,11].
The place of office hysteroscopy
Office hysteroscopy has expanded the role of hysteroscopy in patients with submucous fibroids. Almost all the parameters that were assessed using in patient hysteroscopy can now be evaluated in the outpatient department. The use of outpatient operative techniques like the OPIUMM technique have made treatment of deeper myomas easier and safer [8].
Conclusion
The diagnosis of uterine myomas is accurately defined by imaging techniques thus allowing diagnostic hysteroscopy to be scheduled within the same session as surgery. It is important for every practitioner to grow awareness of the classic hysteroscopic features of myomas which are particularly helpful when differential diagnosis is suspected. In the case of myomectomy where the tumour is enucleated and left inside the cavity, diagnostic hysteroscopy additionally allows biopsy for histopathological confirmation.
References
Figure 1. Correlations between 3D ultrasound and diagnostic hysteroscopy. 2 large submucous myomas located in the corpus and in the fundus with different surface vascularization. Images by A. Drizi
Figure 2. Vascular architecture over fibroid. Image by S. Pisat.
Figure 3. Submucous myoma located next to 2 endometrial mucous polyps: different hysteroscopic features. M: myoma; P: Polyp; 1: first; 2: second; μp: micropolyp. Images by A. Drizi
Figure 4. Endometrial polyp. By S. Pisat.
Figure 5. Endometroid adnenocarcinoma. Image by S. Pisat.
Table 1. The STEP W classification
Figure 6. Large intra cavitary fibroid. By S. Pisat.
Figure 7. The aspect of the submucous myoma’s pseudocapsula at hysteroscopy during resection. M: myoma; Pc: pseudocapsula. . Images by A. Drizi
Figure 8. Submucous fibroid appearing as a type 3 at hysteroscopy. By S. Pisat.
Figure 9. Submucous fibroid type 2 to 5, laparoscopically removed. Image by S. Pisat.
Figure 10. Submucous myoma displaced outward due to distension pressure. By S. Pisat.
Figure 11. Fibroid bulging inward with reduced distension pressure. Image by S. Pisat.