Authors / metadata
DOI: 10.36205/trocar1.2023005
Abstract
The Authors recall how the vagino-scopic approach to hysteroscopy can highlight macroscopically evident alterations of the vagina and the uterine cervix. The use of the Hamou micro-colpo-hysteroscope allows cellular observation “in vivo”, thus allowing an immediate diagnosis of the gravity, the location and the extent of any lesion. The personal experience of the last 42 years with the use of micro-colpo-hysteroscopy and the advantages related to the technique is reported.
Introduction
Diagnostic hysteroscopy gained considerable momentum when – in 1980 – Jacques Hamou conceived and developed his first Microcolpohysteroscope made by Karl Storz SE & Co KG Tuttlingen Germany.
Until then it was a method reserved for very few gynecologists, which required general anesthesia and the use of the operating room. The reduction of the caliber of the instrument, the improvement of vision thanks to the Hopkins lenses, the distention with gaseous medium (CO2) and the enthusiasm of some pioneers, mainly in Italy, contributed to the spread of the diagnostic method.
Starting in the late 1980s, some of us thought of using the so-called “vaginoscopic approach” to the cervical canal and uterine cavity. In particular, to carry out the exploration of the uterine cavity in women with intact hymen it was decided to use a liquid distention medium to observe the vagina without the use of the vaginal speculum, and – subsequently – proceed through the external uterine orifice in the journey discovering the uterine cavity.
In the following years there were numerous improvements characterized by the further decrease in the diameter of diagnostic hysteroscopes and by surgical techniques that allowed the removal of endo cavitary neoformations, as well as the treatment of some congenital malformations.
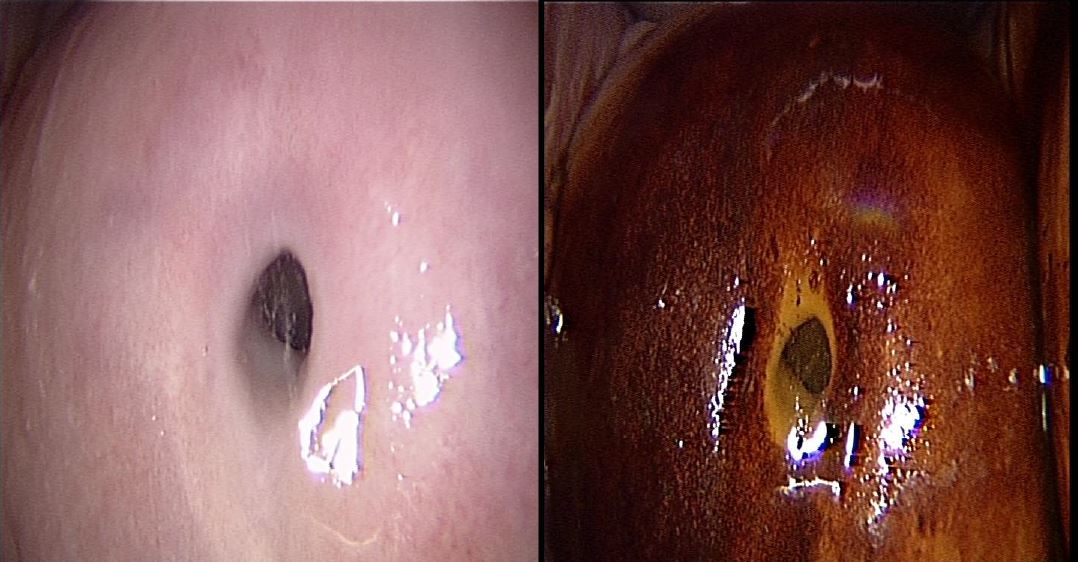
Office hysteroscopy allows magnified examination of the vagina, the ectocervix as well as the cervical canal. Both the vaginal and ectocervical mucosa are lined with a non- keratinized stratified squamous epithelium and appear in a pale pinkish color. Unlike the ectocervix, the vaginal lining adjusts to the cavity by forming big foldings, vaginal wrinkles, until the fornix. To unfold the latter, a moderate distension is needed by a gentle obstruction of the vulvar orifice. The cervix is located at the vaginal dome and can display normal or abnormal features which are visible in hysteroscopy: mucus, ectropion, ectocervical polyps, cysts, adhesions and endometriosis implants, to name but a few (Fig 1-3).
The endocervical canal however is lined by a different mucosa consisting of a single layer of mucus-producing columnar cells, and hence appears more reddish. Among the frequently encountered lesions: Nabothian cysts, cervicitis, polyps and adhesions.
Using the Hamou micro-colpo-hysteroscopy, instead of the normal hysteroscope, we can observe not only the vagina, the cervical canal and the uterine cavity, but also the “contact” vision of the epithelium up to 150 magnifications.
The Waterman’s blue ink (the common ink of fountain pens) can color the squamous epithelium of the uterine cervix and vagina “in vivo”, thus allowing observation without the need to remove the cells and send them to the laboratory.
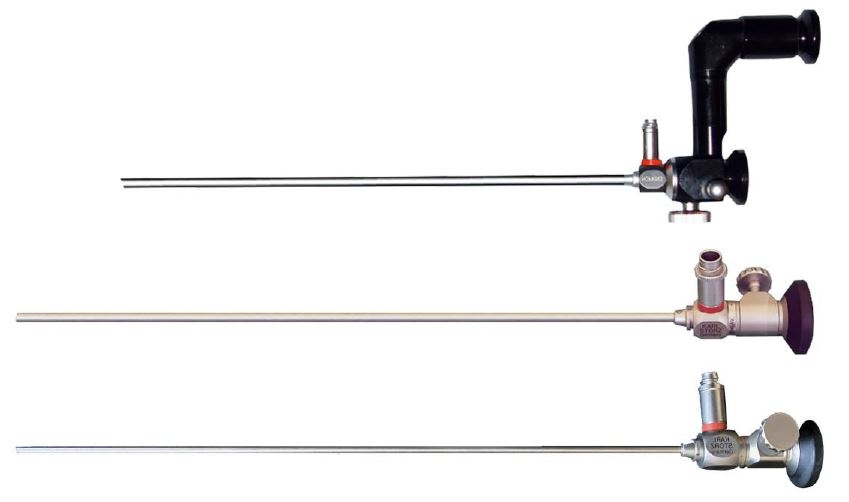
The first micro-colpo-hysteroscope sold in the world was purchased in 1981 by Luigi Montevecchi from Italy and allowed correlation studies between hysteroscopy and histopathology. Over the past 42 years one of the authors (L.M.) has used, in sequence, the three Hamou micro-colpo-hysteroscopes models (type I, II and III) to observe the epithelium of the uterine cervix and vagina for the accurate diagnosis of lesions caused by the Human Papilloma Virus (HPV) (Fig.4).
After the original model, characterized by a double eyepiece and a button capable of diverting – thanks to the interposition of a mirror – the vision from the direct to the offset eyepiece, Hamou created a second model (type II) much more manageable, with only eyepiece that allowed magnification up to 80x. The focus knob (present either on the first model no longer in production, and on the two most recent) allows a permanent clear view at different focal lengths.
The Type III differs from the Type II by the reduction of the caliber which goes from 4.5 to 2.7 mm. It is necessary to have a light source with a fiber optic cable, and a high quality endo-camera to obtain an adequate view of the cellular elements on a large monitor.
Materials and Method
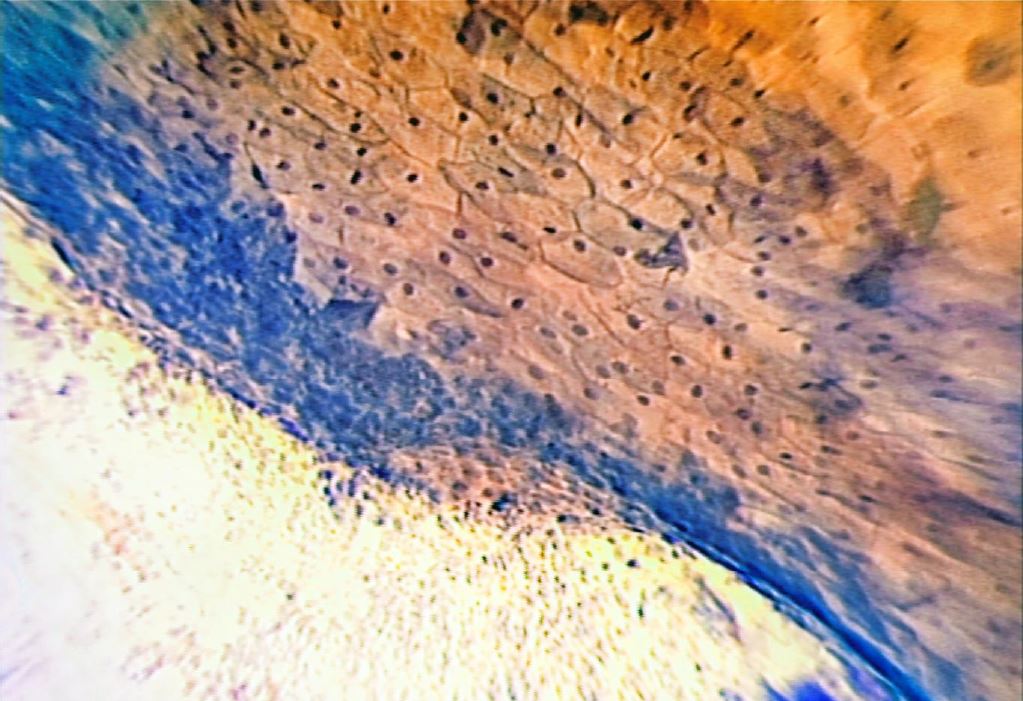
The technique of Microcolpohysteroscopy (MCH), already extensively described in previous publications, consists of exposing the uterine cervix with a common vaginal speculum (1-3). We then proceed to cleanse the surface of the epithelium with a cotton swab soaked in physiological solution, to remove the cervical mucus which can sometimes be very abundant. Lugol’s iodine solution is then applied to assess the degree of maturity of the epithelium. The areas that capture the solution are those stimulated by estrogens, rich in glycogen, and capable of carrying out the normal functions of the mature squamous epithelium. Their color turns deep brown (Fig. 5)
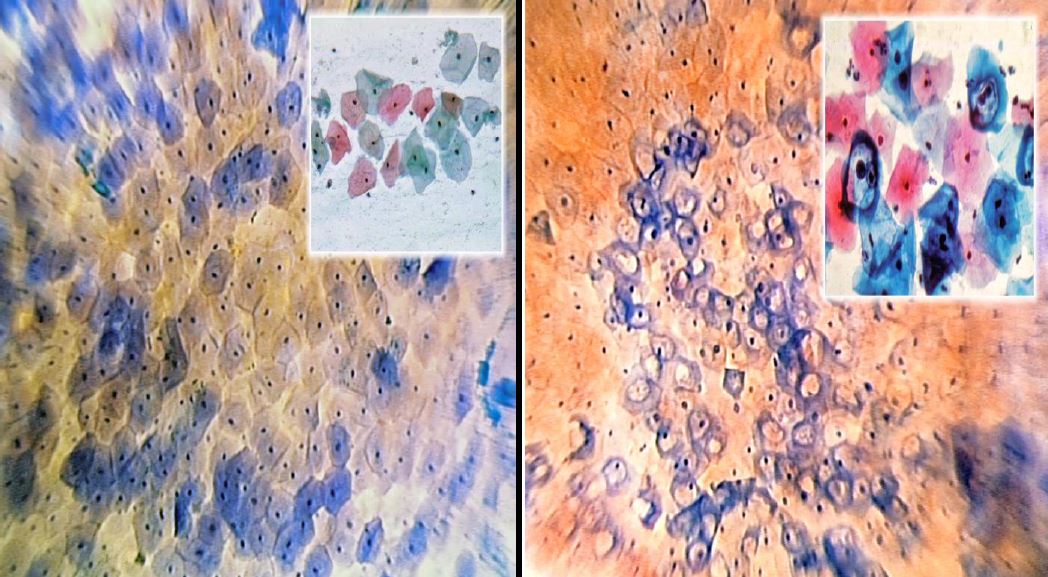
After this first superficial evaluation, the tissue is colored with Waterman’s blue ink. It penetrates into the cytoplasm and the nucleus of the mature squamous epithelium, and into the one in squamous metaplasia (more or less mature), leaving the cylindrical epithelium colorless (Fig. 6). This feature allows an easy differentiation between squamous and cylindrical epithelium, making it easy to identify the squamous-cylindrical junction, so important for the study of pre-neoplastic lesions of the uterine cervix.
We collected most of the data in a digitized way, first on a Personal Computer (PC) with the “Access” database, then, starting from 2012, on a Mac platform with the FileMaker database.
After the first years, during which the MCH images were carefully compared with the results of the histological diagnosis deriving from the biopsy, we stopped carrying out a systematic check, having obtained an almost total correspondence between the MCH diagnosis and the corresponding cytohistological condition, more precisely than the traditional colposcopy and confirmed by flow cytometry (4-7).
Figures 7-9 document in a very precise way the morphological correspondence between normal squamous epithelium, cytopathic alterations from HPV and high-grade lesions (7-10), which allowed us to make an accurate diagnosis without the need to take biopsy samples for confirmation.
Results and Discussion
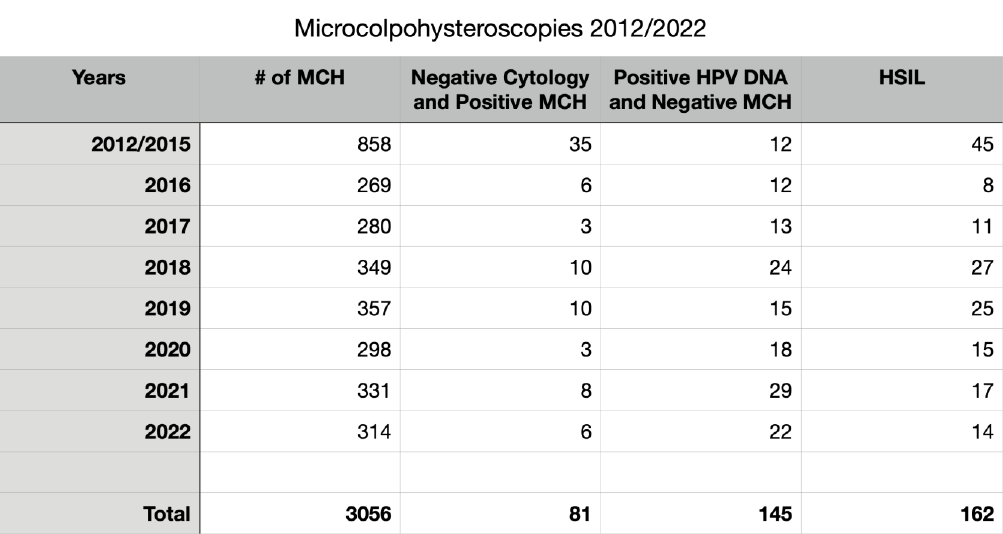
We reviewed 3056 consecutive MCHs performed from 2012 January 1st to 2022 December 31th (Mean age = 41; Median = 39; SD= 12). Some data are summarized in table 1 and Fig 10.
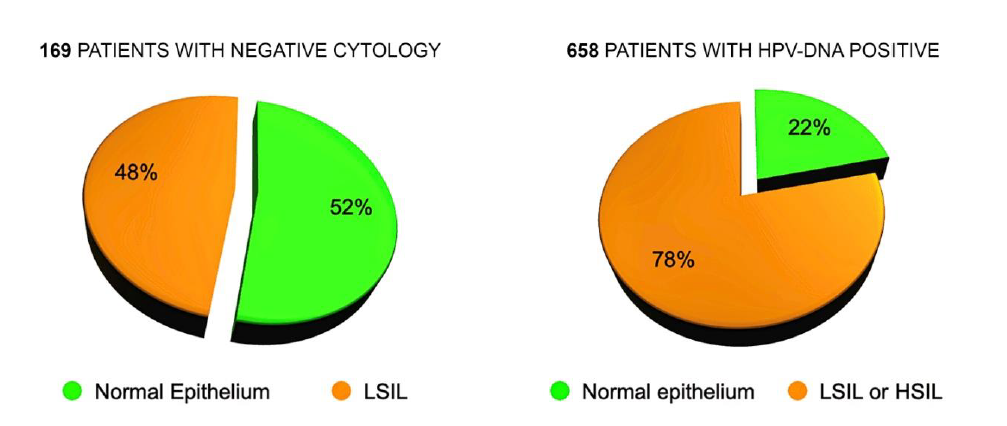
In 169 patients submitted to MCH with negative Pap smear (referred for examination for routine control or for positivity to the HPV DNA test) we diagnosed 81 subjects (48%) with cytopathic alterations caused by the Papillomavirus, not recognized by cytological examination.
This confirms some observations already published in old articles (2) and the reason is the more complete and precise examination of the entire uterine cervix, and of the Squamo-Columnar Junction (SCJ), made by MCH, in comparison with the cellular sample collected with the Ayre’s spatula. In 145 cases out of 658 (22%), sent to MCH for positive Human Papilloma Virus (HPV) test (16, 18, 31, 33 or other Papillomaviruses) there were no cellular alterations referable to the presence of the HPV. This can be easily explained by remembering that the cellular modifications depend on two competing factors: the aggressiveness of the virus and the defense capabilities of the host organism. The presence of the virus, revealed by gene amplification on a swab, is not always capable of inducing cellular alterations detectable by microscopic observation (Fig. 10).
We have diagnosed or confirmed 162 cases of HSIL, identifying the location and extent of the lesions. Most of them have been referred to their own doctor for excisional treatment of the pathological areas. In 16 cases out of 162 we had a copy of the histological examination after conization. The correlation between MCH and histological diagnosis was 100%.
Conclusion
The vaginal approach to the uterine cavity using a liquid distension medium allows to observe normal and pathological aspects of the vagina and uterine cervix, under macroscopic magnification. The microcolpohysteroscopy, performed with the Hamou microcolpohysteroscope, allows immediate observation of the cells lining the vagina and the uterine cervix, without the need to perform a biopsy and wait for the result of the histology.
It allows, in a single session, to determine the presence of a lesion, to evaluate its severity, location and extension and to decide whether to repeat the examination after some time, or to perform a treatment, knowing in advance the location and extent of the lesion. Compared to cytology, it allows identification of the position and extent of altered cells, and eliminates the interval between sampling and the laboratory response. Compared to colposcopy, it allows precise determination of the internal limit of the lesion even in the case of unsatisfactory colposcopy, so as to obtain a customized surgical removal and reduce the risk of excessive or insufficient excisional treatment (12).
Compared to the HPV DNA test – which can only ascertain if a high-risk virus is present or not – it can verify whether – whatever the type of HPV present – it has already been able to induce cellular alterations, and of what degree. Although the term hysteroscopy etymologically indicates examination of the uterus, more can be screened thanks to this technology, including the vagina and the cervix.
Microcolpohysteroscopy, in addition to standard hysteroscopy, allows an excellent in vivo examination of cells which this study has confirmed.
References
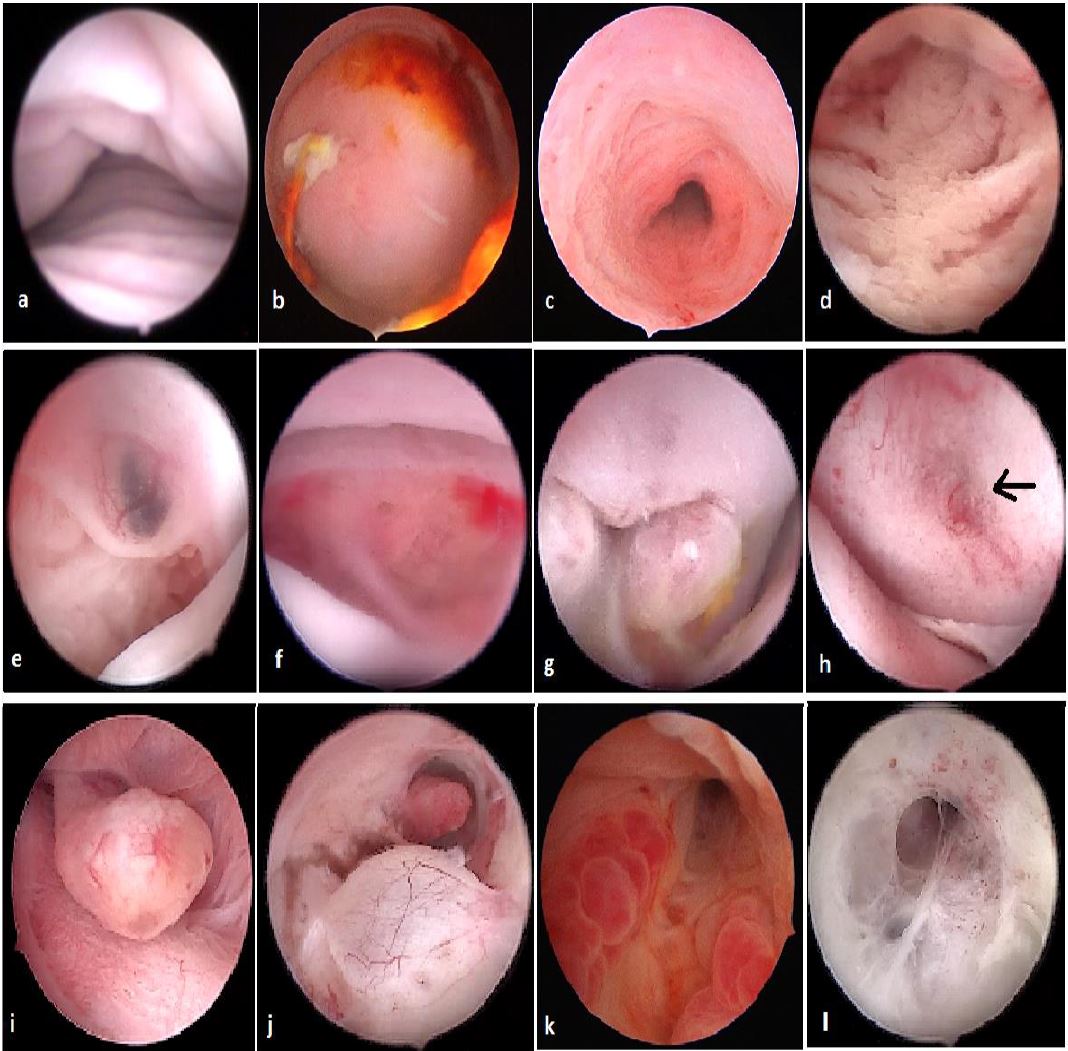
Figure 1. Vagina and cervix at standard diagnostic hysteroscopy. a) vaginal folds; b) ectocervix with mucus; c) cervical canal; d) detail of papillary structures (arbor vitae); e) ectocervical endometriosis; f) ectropion; g) endocervical polypoid papillae; h) total synechia of the external os of the cervix; i) endocervical polyp; j) Nabothian cyst with endocervical adhesions and polyp behind; k) endocervicitis; l): endocervical adhesions. (Images by A. Drizi)
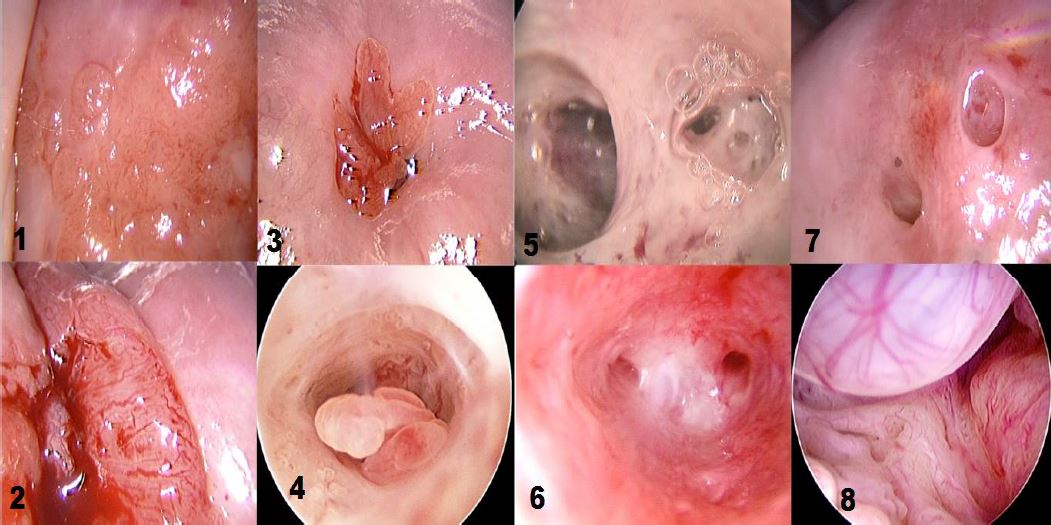
Figure 2. Various cervical lesions: 1) Cervical carcinoma; 2) Adenocarcinoma; 3) Small polyp protruding from external orifice 4: Endocervical polyp 5: Isthmic adhesions 6: Cervical adhesions 7: External orifice adhesions mimicking two orifices 8: Endocervical Nabothian cyst. (Images by L. Montevecchi)
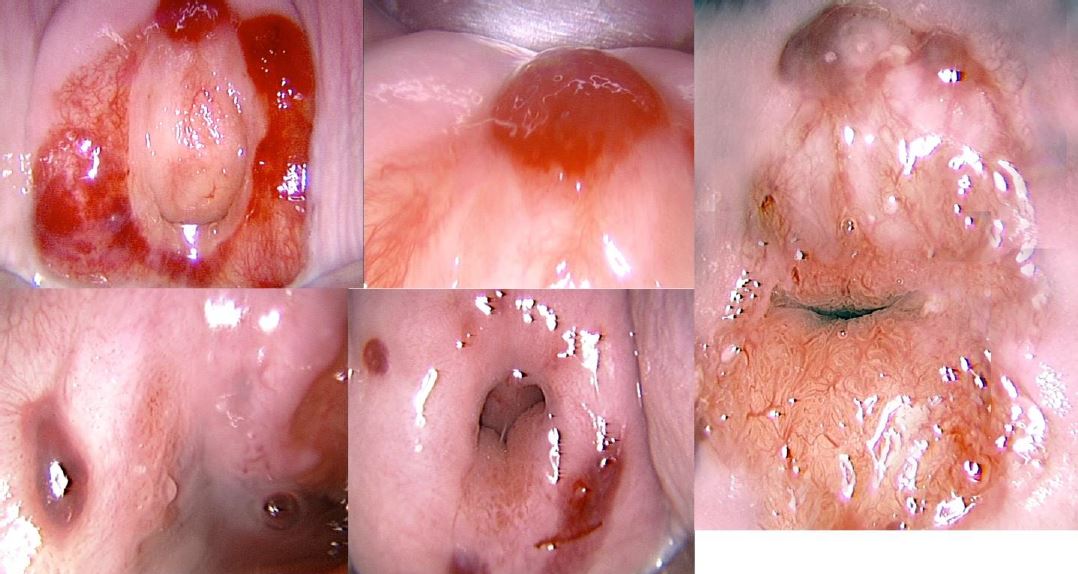
Figure 3. Different aspects of cervical endometriosis. (Images by L. Montevecchi)
Figure 4. From up to down: types I, II and III Hamou microhysteroscopes, with a side focus knob for all models
Figure 5. Normal uterine cervix before (left) and after (right) application of Lugol’s solution. (Images by L. Montevecchi)
Figure 6. The squamo-columnar Junction (SCJ) (Microcolpohysteroscopy x150): Bottom left: unstained cylindrical epithelium; top right: mature superficial squamous epithelium; blue cells: Transformation Zone (Images by L. Montevecchi)
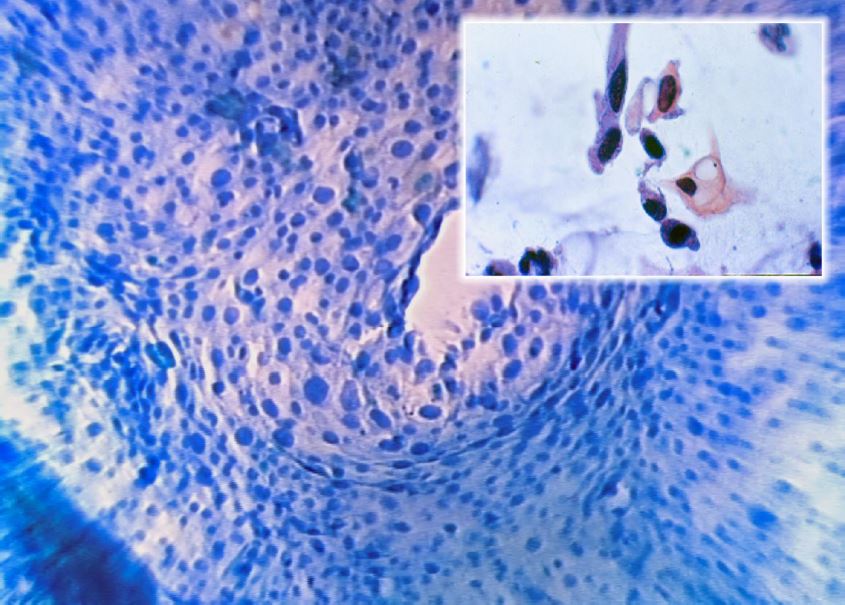
Figure 7. Normal Epithelium (Microcolpohysteroscopy x150): note the polygonal cells with a pyknotic nucleus regularly distributed on the surface of the epithelium (Inset: Cytology x250). Images by L. Montevecchi
Figure 8. Koilocytosis (Microcolpohysteroscopy x150): rounded cells with hyperchromic nucleus and clear perinuclear cytoplasmic halo (Inset: Cytology x250). Images by L. Montevecchi.
Figure 9. High Grade Lesion (Microcolpohysteroscopy x150): marked anisokaryosis, irregular cell distribution, altered nucleus/cytoplasmic ratio in favor of the nucleus with little cytoplasm (Inset: Cytology x250). Images by L.Montevecchi
Table 1. The main findings collected out of the 3056 microcolpohysteroscopy (MCH) procedures from 2012 to 2022. HPV: Human Papilloma Virus; DNA: Deoxyribonucleic Acid; HSIL: High grade squamous intraepithelial lesion.
Figure 10. Discrepancy between Cytology, HPV-DNA Test and Microcolpohysteroscopy. HPV: Human Papilloma Virus; DNA: Deoxyribonucleic acid; HSIL: High grade squamous intraepithelial lesion; LSIL: Low grade squamous intraepithelial lesion.