Authors / metadata
DOI: 10.36205/trocar6.2025001
Abstract
Laparoscopy revolutionized gynecological surgery by enhancing precision, reducing recovery time, and minimizing hospital stays. However, as surgical demands grew, traditional twodimensional laparoscopy revealed limitations, particularly in-depth perception and dexterity. The advent of robotic-assisted laparoscopic surgery has transformed the field, offering improved visualization, tremor reduction, superior ergonomics, and enhanced precision. Over the past two decades, robotic platforms have expanded the scope of minimally invasive surgery. Robotic surgery has transformed gynecological care by enhancing precision, visualization, and surgeon ergonomics. It has expanded the scope of minimally invasive surgery, particularly for complex conditions like advanced endometriosis and gynecological malignancies. As technology advances, addressing cost and accessibility will be key to maximizing its global impact.
Introduction
Conventional two-dimensional laparoscopy was considered a game changing tool, for both the surgeon and patient in terms of surgical precision, enhanced vision, quicker post operative recovery and shorter hospital stays. However, the technology came with its limitations as the scope and skills of surgeons kept growing. The robotic platforms have added to the surgeon’s armamentarium, enabling them to overcome these limitations.
Robot-assisted laparoscopic surgery has become a cornerstone of modern gynecology, offering improved depth perception, reduction in tremor resulting in unparalleled precision, superior dexterity, intuitive movements, autonomy of camera control, improved ergonomics, and hence has expanded capabilities for minimally invasive management of complex gynecological conditions. Over the past two decades, robotic platforms have redefined the surgical approach to challenging diseases such as advanced endometriosis, gynecological malignancies, and pelvic organ prolapse, delivering better outcomes while minimizing surgical morbidity. The evolution of robotic systems, particularly innovations such as Indocyanine Green (ICG) fluorescence imaging and augmented reality (AR), has further enhanced visualization, surgical planning, and multidisciplinary collaboration. This article provides a comprehensive review of the role of robotic surgery in gynecology, focusing on evidence-based outcomes, economic considerations, emerging technologies, and global perspectives, supported by systematic reviews and clinical studies.
History of Robotic Surgery
Although the term and existence of “robots’’ is relatively new, the idea of autonomously operating machines can be dated for centuries. The term “robot” was conceptualized by Joseph Capek in 1921, in his play Rossom’s Universal Robots, which originally came from the Czech word “robota”, meaning “labor”. The term rapidly became corrupted to reflect a machineoriented repetitive task. Computer assistance, robotics, automation, and virtual reality are relatively new concepts, and more recently they have been applied to healthcare assistance. The last decades have witnessed an exponential growth in medical technology (1).
Robotic applications to surgery started in 1970s as military projects endorsed by the National Aeronautics and Space Administration (NASA) and funded by the Defense Advanced Research Project Administration (DARPA) in order to replace the surgeon’s physical presence and provide care to astronauts or to soldiers in battlefields (2). Robotic surgery represents a pinnacle in minimally invasive techniques, integrating cutting-edge technology to enhance surgical precision. The evolution of robotic surgery can be traced back to its inception in the 1980s. The “da Vinci Surgical System” by Intuitive Surgical (Intuitive Co Sunnyvale, California, USA), introduced in the early 2000s, marked a watershed moment. Understanding the key components of robotic surgical systems is imperative for appreciating their efficacy in surgical management.
Surgeon’s Console: The control center where the surgeon sits and manipulates the robotic arms with hand and foot controls.
Robotic Arms: Articulating arms equipped with surgical instruments, allowing for precise movements during the procedure.
Endoscope and 3D Camera System: High-definition, three-dimensional visualization is provided by the endoscope, enhancing the surgeon’s depth perception.
Patient Cart: The robotic system’s mechanical arms and instruments are housed in the patient cart, which is positioned next to the operating table. I Evidence-Based Outcomes in Robotic Surgery Robotics in Benign Gynecology.
Robotic myomectomy
The surgical steps and concept of abdominal myomectomy was first defined in 1931. The same was described using minimally invasive techniques (conventional laparoscopy) in 1979. The success of minimally invasive techniques served as the foundation for the development of robotic myomectomy (RM), which was accepted by surgeons. Since robotic platforms offer many benefits, are more intuitive, not requiring all the skills that are needed for conventional laparoscopy, they are particularly advantageous to surgeons with few or no laparoscopic expertise, particularly in suturing procedures. Myomectomy is a suture-intensive procedure, and the aid of a robotic platform reduces the learning curve for intra-corporeal suturing (3). One of first series of myomectomy that was reported in the literature using the Da Vinci robot was by Advincula et al., in 35 patients (4). The mean diameter of fibroids was 7.9 ± 3 cm, mean weight was 223 ± 244 g, and each patient had an average of 1.6 fibroids removed at the time of surgery. The conversion rate from robotic to laparotomy was 8.6%, comparable to that of conventional laparoscopic myomectomy. The study reported mean estimated blood loss to be 169 ± 198 ml with average operative times of 230 ± 83 min (5).
The pseudo capsule surrounding the fibroid is a fibro-neurovascular structure composed of a neuro-vascular network rich in neuro-fibers separating it from normal peripheral myometrium. The fibroid pseudo capsule is similar to the neuro-vascular bundle surrounding a prostate. This understanding of the neurovascular bundle in pseudo-capsule has brought in a concept of intra capsular fibroid nerve-sparing laparoscopic “microsurgery,” or intra capsular fibroid nerve-sparing robotic-assisted “nanosurgery,” with the help of robotic magnification.
Intra capsular myomectomy preserves the neuro-vascular bundle and neurotransmitters surrounding fibroids. This helps in better healing of myometrium, minimal adhesion and good postoperative scar integrity. Tinelli A. et al, propose that intra capsular myomectomy should always be recommended to maximize the potential for future fertility and to minimize the risk of labor dystocia or uterine rupture during subsequent pregnancy (6).
Robotic Benign Hysterectomy
Hysterectomy is the most commonly performed gynecological surgery in women world over. The first hysterectomy by conventional laparoscopy was described by Harry Reich in 1989 (7). However, 35 years down the line, open abdominal hysterectomy still remains a very common surgical technique. This applies not only to India but to United States of America as well. Most investigators consider higher costs per procedure secondary to lengthier operative time and disposable equipment.
Louis Lenfant et al, made a head-to-head comparison of all routes of hysterectomy for benign conditions: laparoscopic, robot assisted, vaginal and abdominal. It was a systematic review and meta-analysis across various platforms (8).
They concluded that, while the robotic approach was mostly comparable to the laparoscopic route, it was associated with a shorter length of stay, less estimated blood loss, and fewer complications when compared to the open approach. In comparison to the vaginal approach, the robotic route was associated with longer operative time but shorter length of stay and less estimated blood loss. The robotic approach could be an opportunity to offer the peri-operative benefits of minimally invasive surgery to patients who in the past would have been suitable only for an open approach, thus broadening the surgical armamentarium for benign hysterectomy (8).
Robotics in Advanced Endometriosis
Advanced endometriosis remains one of the most challenging conditions to treat surgically. The disease frequently infiltrates critical structures such as the bowel, bladder, ureters, and diaphragm, necessitating complex surgical excision. Robotic platforms have proven particularly effective in addressing these challenges by offering enhanced visualization. The magnified, three-dimensional immersive vision improves the surgeon’s ability to identify and excise microscopic endometriotic implants.
The 3D camera is secured and controlled by the surgeon, reducing dependency on a proficient assistant surgeon. To overcome lack of stereoscopic vision in conventional 2D laparoscopy, 3D cameras were developed. Although the benefits of 3D cameras are well documented, the surgeons’ console vision in robotic-assisted surgery might make the operative strains reported when using a 3D camera in conventional laparoscopy, such as headache, dizziness and eye strain, less severe (9,10).
The improved ergonomics of the robotic platforms cannot be overlooked. Studies have reported the advantages of articulating instruments as well as intuitive movements of the surgeon’s fingers using abdominal models representing a healthy weight range (BMI 18.5–¬ 24.9 kg/m2) and those with obesity (WHO classification BMI 30 kg/m2 or over) (11). Articulated instruments and the intuitive console reduce surgeon fatigue, ensuring sustained precision during long, intricate procedures. In Deep Infiltrating Endometriosis (DIE) specifically, robotic assisted surgery is an enabling tool that may allow surgeons to perform excisions more easily and safely, especially as nerve sparing and focus on the pathology of the pelvic nervous system (neuro-pelveology) becomes imperative (12). In a study, Nezhat et al compared out comes of deep infiltrating endometriosis surgery in 86 patients (conventional laparoscopy) v/s 32 robot assisted surgeries They concluded that despite a higher operating room time, robotic-assisted laparoscopy appears to be a safe minimally invasive approach for patients, with all other peri-operative outcomes, including intra-operative and postoperative complications, comparable with those in patients undergoing conventional laparoscopy (13). The more one can see, the better the result of excision surgery. Mabrouk et al. (2015) reported spontaneous conception rates exceeding 50% following robotic excision in infertile patients, highlighting its efficacy in restoring reproductive function (14).
Deep Infiltrating Endometriosis: Our Perspective & Surgical Technique
This review would be incomplete without sharing our own practical experience on deep infiltrating endometriosis and bowel endometriosis. Deep endometriosis surgery demands a nuanced approach, considering the intricate involvement of pelvic and extra pelvic organs. The surgical techniques employed encompass a spectrum of procedures, each tailored to address the specific challenges posed by deep infiltrating lesions. Excisional surgery, involving meticulous removal of endometriotic tissue, is the cornerstone in the management of deep endometriosis. This may involve:
- Nodule Excision: Targeted removal of deep nodules infiltrating pelvic organs, such as the rectum, bladder, or utero-sacral ligaments (15).
- Peritoneal Shaving: Delicate excision of endometriotic lesions from the peritoneum, preserving surrounding healthy tissue (16).
- Nerve-Sparing Techniques: Preservation of pelvic nerves during surgery to mitigate the risk of postoperative pain and dysfunction (17).
Our technique: The patient is placed in the traditional position for laparoscopic surgery with the legs in Allen stirrups with the minimal amount of flexion at the hip and knee joints.
The primary incision is made at the umbilicus or the Lee Huang point depending on the patient’s body habitus, the size of the uterus in case of a concomitant hysterectomy or the anticipated need of a colorectal resection.
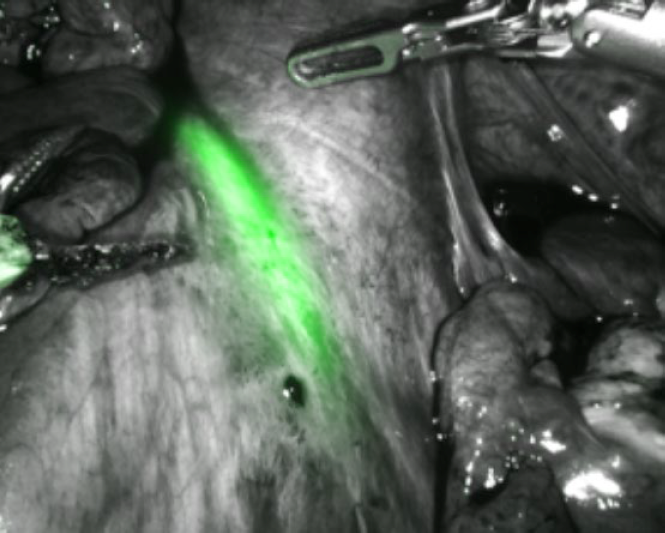
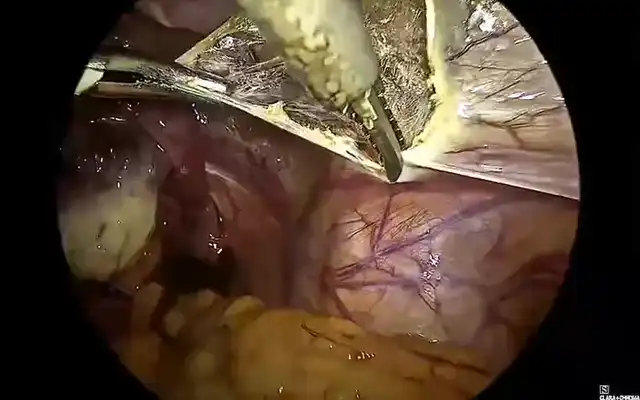
Prior to beginning the procedure, a cystoscopy is performed and both ureteric orifices are identified. Indo Cyanine Green (ICG) dye is injected into both ureters using a ureteric catheter through the cystoscope. The dye will stay in the ureters for approximately four to five hours and will help in identifying the ureters with the da Vinci’s firefly mode, without having the hindrance of stents (Fig. 1).
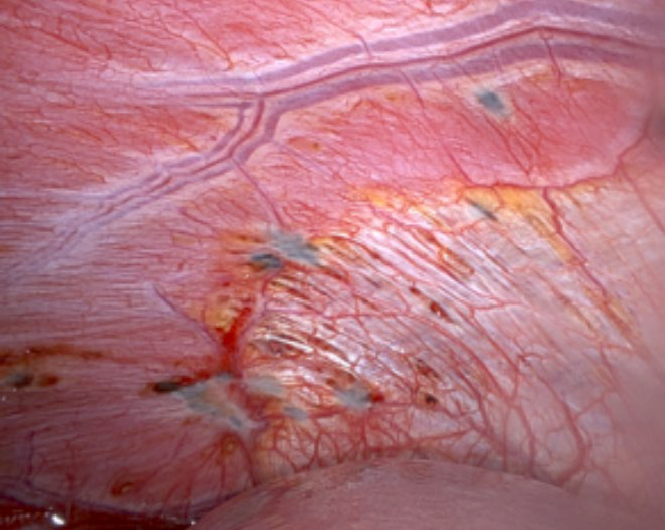
A Veress needle is used to create pneumoperitoneum and an optical entry technique is used with a 5 mm 0-degree laparoscope placed through the optical trocar of the robotic cannula. Once entry is confirmed, the 30-degree robotic telescope is used to perform a thorough inspection of the abdomen and pelvis. Both domes of the diaphragm are closely examined for any endometriotic lesions. It is important to use the angled scope to push down the liver and inspect the diaphragm behind it for any endometriotic lesions (Fig. 2). The rest of the abdomen is surveyed including the stomach, small intestine, cecum, appendix and large intestine.
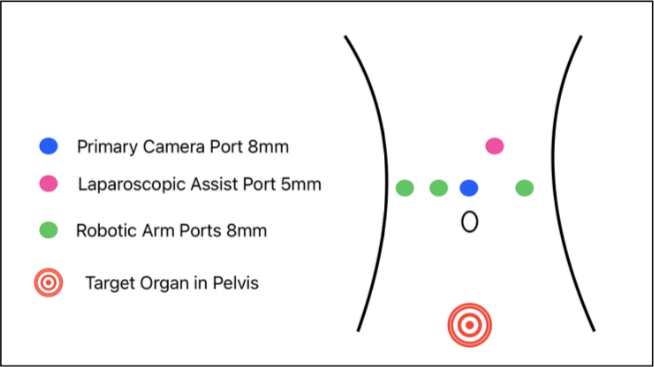
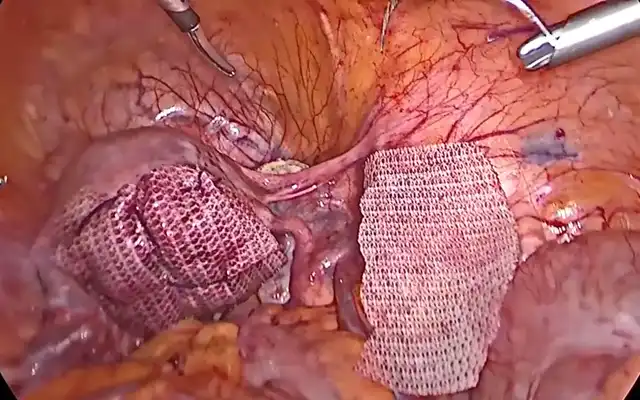
The other robotic ports are placed under vision in a linear fashion. two ports on the right and one port on the left. A 5mm or 12mm assistant port is placed between Arms one and two (Fig. 3).
After port placement the patient is placed in steep Trendelenburg position and the small bowel is pulled out of the pelvis and the uterus is anteverted to enable a complete survey of the pelvis. The robot is then docked from the patient’s right side and instruments are placed. traditionally a long bipolar forceps, monopolar shears and a prograsp for most of the dissection are used.
Preparation begins by mobilizing the sigmoid colon from its congenital adhesions to the pelvic sidewall. This may be performed all the way up to the splenic flexure if a segmental bowel resection is planned. This mobilization gives direct visualization of the left ureter which can be confirmed via the Firefly mode by identifying the bright green of the ICG dye.
Ureterolysis can be performed up to the adherent ovary. In case if an endometrioma; it is drained and decompressed after inevitable rupture during ovariolysis from the pelvic sidewall. Once the ovary is completely mobilized it is elevated and a temporary ovariopexy is performed to the lateral wall using a straight needle suture or a T lift device. The same procedure is repeated on the right side. Ureterolysis is completed after fixation of the liberated ovaries up to the intra-ligamentary portion. Endometriotic lesions form the peritoneum and uterosacral ligaments can now be safely excised. In case of obliteration of the pouch of Douglas by a recto vaginal nodule, one must begin dissection of the para-rectal spaces laterally and then advance along those avascular spaces up to the nodule. In case a segmental resection is planned it is important to enter the avascular Total Meso- Rectal Excision (TMRE) plane to mobilize the bowel. This is a nerve sparing technique which provides direct visualization of the hypogastric nerves and enables us to move them away laterally.
The midline dissection is the trickiest part of the dissection since it involves cutting through the nodule leaving some portion on the uterine torus and some portion on the rectum, both of which are excised after the dissection is completed and the Pouch of Douglas is entered. A speculum examination should have been performed prior to beginning the procedure to rule out infiltration of the vagina. In case vaginal infiltration is present a colpotomy is made and the affected vagina is excised.
Rectal Endometriosis – Preoperative evaluation is a must. Endometriosis surgery requires a thorough and complete evaluation of the rectum. Rectal nodules can be easily diagnosed on a Trans Vaginal Sonography (TVS) or an MRI provided the imaging is performed by or interpreted by an expert. For low rectal nodules, the robotic approach offers us an invaluable advantage. Deep shaving is optimized due to the stable camera and fine instrumentation. In some cases; a low anterior resection may be avoided using the robotic platform as compared to a laparoscopic approach. This may have tremendous advantages for long term patient outcomes as well as reducing complication rates.
For single rectal nodules, less than five cm in length, not infiltrating a circumference more than 1/3rd of the bowel or causing sub-occlusion of the lumen – it is possible to perform a discoid resection with a circular stapler. Shaving is reserved for smaller nodules not infiltrating deep into the muscularis. The limitations with shaving are that it is not a standardized technique and the endpoint is subjective. The recurrence rates may be higher when compared with the other full thickness excision techniques.
Shaving techniques appraisal:
- Excision of rectal lesions without entering the lumen
- Low rate of complications.
- Low rate of functional outcomes or nerve damage
- Long term recurrence is probably higher
- First line technique when feasible
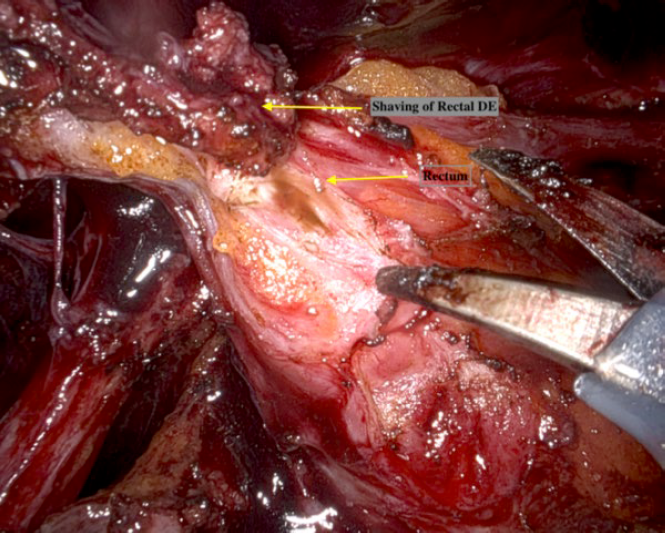
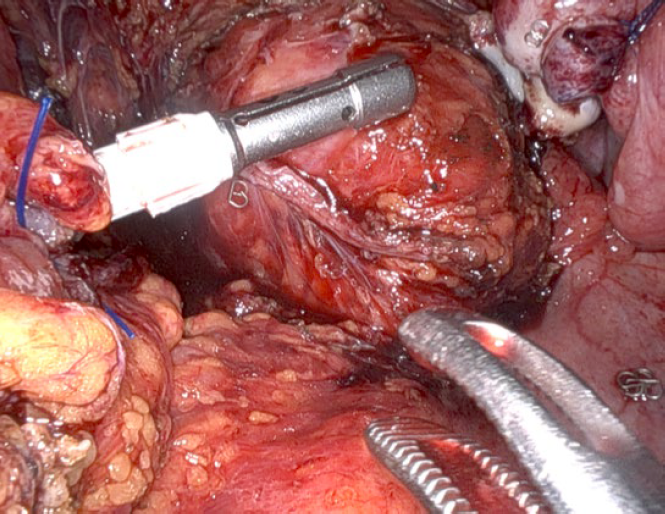
Shaving with the da Vinci robot is much easier when compared with traditional laparoscopic techniques because of the clarity of vision and steadiness of the camera, as well as the fine tipped instruments which allow layer by layer dissection of the rectum and the dexterity of the instruments which allow us to approach the nodule from different directions (Fig. 4).
Discoid Resection: It is an interesting procedure for a full thickness excision of the anterior wall of the rectum. It can be used for single nodules less than four cm in diameter with less than 1/3rd circumference infiltration and/or sub-occlusion. The bulk of the nodule is shaved to reduce the size of the disc. A suture is placed proximal and distal to the nodule. An appropriately sized End to End Anastomosis (EEA) circular stapler is inserted trans-anally with the anvil in place and introduced into the pelvis under vision.
The stapler is then opened and the 2 ends of the suture are used to push the nodule into the open stapler. The stapler is then anteverted and closed ensuring that the nodule remains within and then fired. The stapler is then released and extracted and the specimen is examined to confirm the complete excision of the nodule. Safety tests are performed by insufflation with air and methylene blue / ICG. Segmental Resection: segmental resection anastomoses are reserved for bowel endometriosis in specific conditions:
- Nodules causing sub-occlusion where a discoid excision is not possible
- Single nodules infiltrating a length of more than five cm.
- Multiple nodules in close proximity to each other.
- Nodules extending more than 1/3rd circumference of the bowel.
- Sigmoid colon nodules.
- Small bowel or ileo-caecal endometriosis.
Procedure
When feasible segmental resection and anastomosis is carried out via Natural Orifice trans-luminal Specimen Extraction techniques (NOSE). These can be performed via three routes
- Trans-vaginal – When performed in conjunction with a hysterectomy the open vagina can be used to introduce the anvil and as a route to extract the specimen. In some cases, it may be feasible to exteriorize the affected bowel through the open vagina but if this is not possible then the procedure can be completed easily enough via an intra-corporeal technique.
- Trans-colpotomy – In cases when there is vaginal infiltration and a vaginal nodule must be excised the posterior colpotomy resulting from this procedure can be used to introduce the anvil as well as extract the specimen.
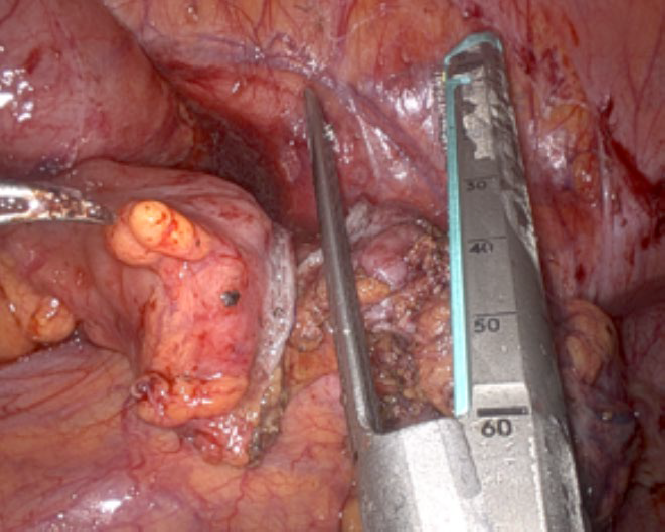
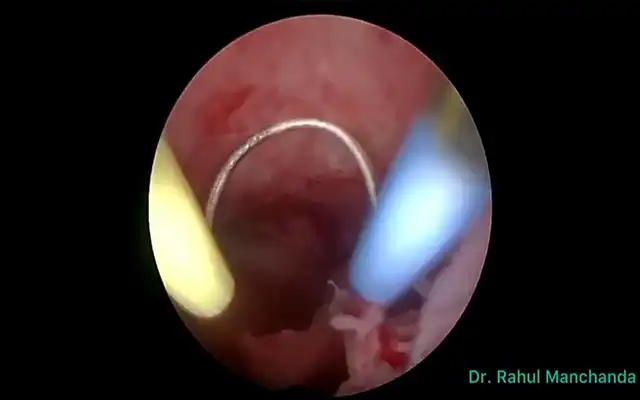
- Trans-anal approach – In cases where there is no vaginal infiltration and there is no simultaneous hysterectomy being performed an enterotomy is made proximal and distal to the affected length of bowel. The anvil of the EEA stapler with the spike in place is introduced via the caudal enterotomy (Fig. 5). The anvil is then introduced through the cranial enterotomy and the spike is maneuvered to pierce the anti-mesenteric border of the proximal colon. A linear stapler is used to disconnect the bowel below the anvil. The spike is then disconnected and retrieved by the bedside assistant port (Fig. 6). The resected bowel is then disconnected and the specimen is retrieved through the distal rectum which is then staple closed. Finally, the side to end anastomosis is performed as per standard procedure.
Robotics in Gynecological Oncology
Robotic surgery has become a mainstay in the treatment of gynecological malignancies, particularly in endometrial and cervical cancers. Women with endometrial cancer often present with co-morbidities, such as severe obesity, diabetes or hypertension (18). The platform’s ability to facilitate comprehensive staging,
including lymphadenectomy, while minimizing surgical morbidity has made it a preferred choice for both patients and surgeons. Corrado et al. (2018) analyzed over 5,000 cases of robotic hysterectomy, reporting oncologic outcomes comparable to open surgery with significantly reduced complications, shorter hospital stays, and faster recovery (19). Over the past two decades Minimally Invasive Surgery (MIS) techniques have gained widespread acceptance as an approach to radical hysterectomy for cervical cancer (20). A meta-analysis assessing robotic assisted surgery for radical hysterectomy in cervical cancer concluded there were fewer complications using the robotic platform compared with open surgery (21). Nie JC et al. (2017) noted that robotic radical hysterectomy in early-stage cervical cancer led to superior perioperative outcomes compared to laparotomy, including lower wound infection rates and reduced blood loss (22).
Robotics in Pelvic Organ Prolapse Surgery
Pelvic Organ Prolapse (POP) significantly impacts a woman’s quality of life and robotic-assisted sacrocolpopexy / Sacro hysteropexy are now emerging as the gold-standard surgical approach for advanced cases. However, the role of the robotic platform has expanded to include colposuspension, management of vesico-vaginal fistula and mesh/suture complications (23). The robotic platform provides greater precision and access to the deeper spaces and planes in the pelvis. Most Urogynecological surgeries being suture intensive, the seven degrees of freedom along with the articulating instruments are definitely favorable to enhance surgical experience. The superior ergonomics result in lower error rates and shorter learning curves for Urogynecological procedures (24,25). Paraiso et al., 2017 demonstrated long-term success rates exceeding 90%, with lower rates of prolapse recurrence and mesh erosion compared to vaginal approaches (26). Kim JH et al. (2020) demonstrated fewer intra-operative complications and faster recovery time with robotic sacrocolpopexy compared to laparoscopic techniques (27).
II Innovations in Visualization: Indo-cyanine Green and Augmented Reality
(a) Indo-Cyanine Green (ICG) Imaging
ICG fluorescence imaging has become an integral component of robotic gynecological surgery, enabling superior visualization of critical structures and reducing rates of surgical complications. Ureteral Visualization: Conditions such as deep infiltrating endometriosis and patients who have had recurrent disease requiring multiple surgeries have altered surgical anatomy and poor surgical planes for dissection. The power of ‘predictive anatomy’ cannot be applied in such surgeries. There in comes the use of intra-ureteric instillation of ICG which allows the surgeon to visualize the entire ureter along its course during crucial dissection to restore normal anatomy. ICG allows precise identification of the ureters during complex pelvic surgeries, reducing the risk of iatrogenic injury. Yoshida et al. (2019) reported that ICG reduced ureteral injury rates by 25% in gynecological surgeries (28).
By giving an intravenous bolus ICG can be used to confirm good vascular supply to the ureter after a difficult ureterolysis; ruling out thermal / ischemic damage.
•Bowel Anastomosis: an intravenous bolus of diluted ICG helps identify vascular perfusion post dissection and / or anastomosis. It is used in cases of bowel endometriosis after resectionanastomosis of the recto-sigmoid. It ensures adequate perfusion at the anastomotic site which is critical in bowel resections. ICG imaging confirms vascular supply in real-time, reducing anastomotic leak rates. Chan D. K. H. al. (2020) found that using ICG in robotic bowel resections lowered anastomotic leak rates by 50% (29).
•Sentinel Node Mapping: ICG enables real-time identification of sentinel lymph nodes (SLN) in gynecological cancers, minimizing the need for extensive lymphadenectomy and reducing complications such as lymphedema. Paracervical instillation of diluted ICG just prior to starting the surgery, helps identification of the sentinel lymph node. This in turn reduces the morbidity of the surgery, operative time and need for prolonged anesthetic medications. Jewell et al. (2014) demonstrated a 95% detection rate for SLNs using ICG in endometrial and cervical cancer surgeries (30).
(b) Augmented Reality (AR)
AR technology offers a transformative addition to robotic surgery, allowing real-time integration of anatomical maps with the surgical field.
•Enhanced Surgical Planning: Surgeons can visualize critical structures such as blood vessels, ureters, and lymph nodes during surgery, improving safety and efficiency. This avoids inadvertent iatrogenic damage to vital tissues and augments the principle of vessel sparing and nerve sparing surgery.
•Dynamic Intra-operative Guidance: AR overlays guide surgeons to key anatomical landmarks, such as sentinel nodes, while avoiding unnecessary dissection. This not only reduces the morbidity of the surgery but also shortens surgical time and avoids need for prolonged anesthetic medications.
•Integration with ICG: Combining AR with ICG fluorescence provides unparalleled visualization, enabling real-time confirmation of tissue perfusion, lymphatic mapping, and tumor margins. As AR technology matures, its integration with robotic systems is expected to further improve outcomes, reduce operative times, and enhance safety in complex gynecological procedures.
III Multidisciplinary Collaboration
Robotic systems provide a unique platform for seamless multidisciplinary collaboration, particularly in cases involving advanced endometriosis. The enhanced precision and stability make robotic surgery particularly suited to operating in areas with challenging access, such as the pelvis, which has led to a rising number of colorectal resections being performed robotically (31). Robotic platforms facilitate precise ureterolysis and bladder repair, which can be challenging with conventional laparoscopy. Urologists have taken on the robotic platform and are more comfortable suturing robotically than with conventional laparoscopy. The articulated instruments and consistent surgical setup enable efficient bowel resections and anastomoses, even in the narrow confines of the pelvis. Colorectal surgeons are using robotic staplers which articulate and enable lower resections of the recto-sigmoid.
As coordinators of care, gynecologists leverage the robotic system to manage the primary disease while ensuring effective contributions from other specialists. Farr Nezhat et al. (2023) reported improved outcomes in advanced endometriosis surgeries performed collaboratively with urologists and colorectal surgeons using robotics, with significantly lower complication rates (33).
IV Post Operative Pain Relief & Analgesia Management
Despite the port sites being 8 mm in robotic surgery, the port site pain is expected to be lesser than conventional straight stick laparoscopic surgery. This may be due to less torquing at the level of the abdominal wall through the operative ports as majority of the movement happens within the abdominal cavity at the tip of the instrument (endo-wrist movement) In conventional laparoscopy, the abdominal wall port site is used as the point of fulcrum for all movements, resulting in greater post operative pain. Shashoua et al (2009)evaluated narcotic usage and found that the robotic procedures required fewer units of post operative narcotic usage (34).
V Economic Considerations
Robotic surgery is frequently criticized for its high costs, but evidence suggests that its long-term value may offset these initial expenses. It is important to consider that costs should decrease with time once surgical expertise increases (shortened operating time), high-volume robotic centers are introduced, hospital stays reduce and MIS increases. Also, the cost and time implications associated with laparotomy complications should also be considered (35-37). While one debates and questions the justification of added costs, one must consider the long-term value over short term cost. Wright et al. (2019) found that robotic surgery reduced postoperative complications and hospital readmissions, lowering overall healthcare costs by 15% over one year (38). As robotic surgery spreads its arms across intuitions and multidisciplinary fields, one can expect improved efficiency in high-volume centers. Sandberg et al. (2020) noted that centers performing high volumes of robotic procedures achieve better cost-effectiveness due to streamlined workflows (39). Global accessibility of the technology remains a glaring challenge. Low resource settings would face hurdles and barriers in meeting with high upfront costs and this limits the adoption of the robotic platforms in Low- and Middle-Income Countries (LMIC). This can be overcome by the enforcing subsidized programs and developing mobile robotic units that can improve accessibility of the technology.
VI Training and Surgeon Learning Curve
Simulation based training provides surgeons the ability to visualize intra-operative scenarios and troubleshoot through different potential complications enabling them to refine their skills in a controlled and safe environment, increasing their accuracy and gaining crucial experience. Lenihan et al. (2017) studied and then emphasized the importance of standardizing robotic surgery training, combining didactic sessions with hands on simulator or cadaveric practice (32). Currently, the general consensus is that a curriculum should consist of steps ranging from e-learning lectures to simulation and cadaveric training to supervised operating and finally, to approval for independent practice. (32,40,41). The lack of haptic feedback on the robotic platforms; necessitates the development of visual cues which is imperative while transitioning from conventional laparoscopy to robotic assisted laparoscopic surgery. There is debate as to whether previous laparoscopic experience is desirable or necessary before beginning robotic training.
Andolfi and Umanskiy suggest that prior proficiency in the operation itself is important but that whether this is laparoscopic or open makes no difference (42). The endo-wrist technology allows surgical maneuvers that are similar to open surgical techniques, thus making it easy for surgeons with less advanced laparoscopic skills to learn and perform difficult tasks like intra corporeal suturing and knot-tying (43). Studies suggest that 50-70 cases are required to achieve proficiency in robotic surgery, with benefits including reduced operative times and complications over the long term. The availability of a dual console enables collaboration and facilitates teaching (44). A structured training program for robotic surgery is essential. Robotic surgical training programs consisting of two components – generic robotic skills and specialty specific skills – have been developed to introduce Robotic-Assisted Laparoscopy (RAL) programs safely (45). A study by Rajan Babu A, et al showed that robotic surgery has lots of scopes for surgical improvement beyond its first and second years or beyond the first 200 cases. Further evaluation is required to see whether OR times can be reduced further and what is the time /number of cases required for a surgical team to reach its peak performance (46).
VII Future Directions in Robotic Surgery
Artificial Intelligence (AI): A space which can be advanced by AI is in the case of real-time decision support where multiple AI algorithms can guide surgeons in identifying surgical planes, highlighting the involved anatomy and improving precision. Another area which can be improved with AI is the use of predictive modelling, where patient details and scans can be input into the AI algorithm and the output would help predict specific anatomical anomalies and potential complications which would in turn enhance intra operative safety.
Telemedicine and Remote Surgery: Remote surgery has the potential to have the largest impact and is a lucrative topic to take forward as it has the ability to bridge the gap between highly populated, underserved areas with experienced robotic surgeons able to complete complex surgeries from miles away by combining robotic surgery with telemedicine. This would involve high-speed internet connections to enable surgeons to perform procedures on patients in different locations.
Conclusion
Robotic surgery has emerged as a transformative force, revolutionizing the management of complex gynecological and pelvic conditions. It offers unmatched precision, enhanced visualization through innovations like ICG imaging and AR, and facilitates effective multidisciplinary collaboration. Needless to say, better ergonomics result in improved comfort for the surgeon, enabling one to maintain the quality of surgical art for longer hours. These advancements have expanded the scope of MIS, particularly in complex and tedious cases such as advanced endometriosis and gynecological malignancies. By addressing challenges related to cost, training, and accessibility, robotic surgery has the potential to become a universe.
References
Figure 1: Intra-ureteric ICG delineating the right ureter in FireFly mode
Figure 2: Port placement with the da Vinci X Surgical System.
Figure 3: Diaphragmatic Endometriosis seen using a 30° robotic scope
Figure 4: Rectal shaving
Figure 5: Anvil of Circular Stapler
Figure 6: Linear robotic stapler trough a 12 mm robotic port