Authors / metadata
DOI: 10.36205/trocar5.2024023
Abstract
The therapeutic use of lasers in gynecology has developed significantly in recent years. This article summarizes the numerous options and provides an overview of the applications.
Introduction
The use of laser technology in gynaecology has become widespread since the CO2 laser was initially used by Kaplan and colleagues in 1973 for treatment of cervical erosions (1). Since then, many advancements in laser technology have been made, and several other types of lasers are now available, including the neodymium: yttrium-aluminium-garnet (Nd:YAG), potassium-titanyl-phosphate (KTP), and argon. At the same time, the laser has become a popular instrument in laparoscopy, especially in the area of infertility. Despite its popularity in the late 1980s, the early 1990s saw a push to reduce healthcare costs, which led to the adoption of less expensive alternatives like unipolar instruments and loop electrical excision. Despite this shift, laser technology continues to offer unique advantages for gynaecologists.
History
Laser is an acronym that stands for light amplification by the stimulated emission of radiation, a concept that was developed by Einstein in 1917 (2). The first laser developed by Theodore Maiman in 1960 used a ruby as the active medium, and in 1961 the CO laser was introduced (3,4). The CO laser was used in gynaecology for the first time in 1973 for the treatment of cervical erosions, and later by Bellina for the treatment of cervical intraepithelial neoplasia (CIN), as well as for microsurgery of the fallopian tube. The use of KTP, argon, and Nd:YAG lasers became popular in the early 1980s.
Laser Physics
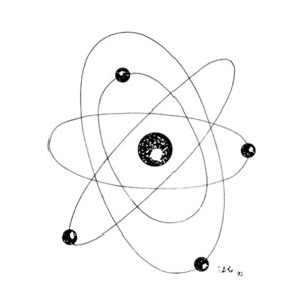
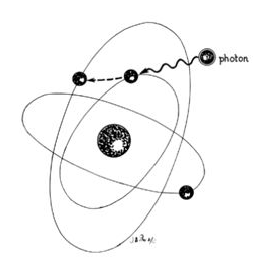
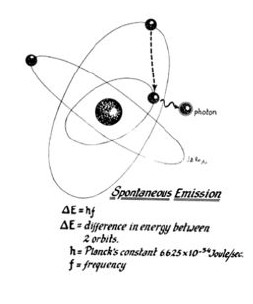
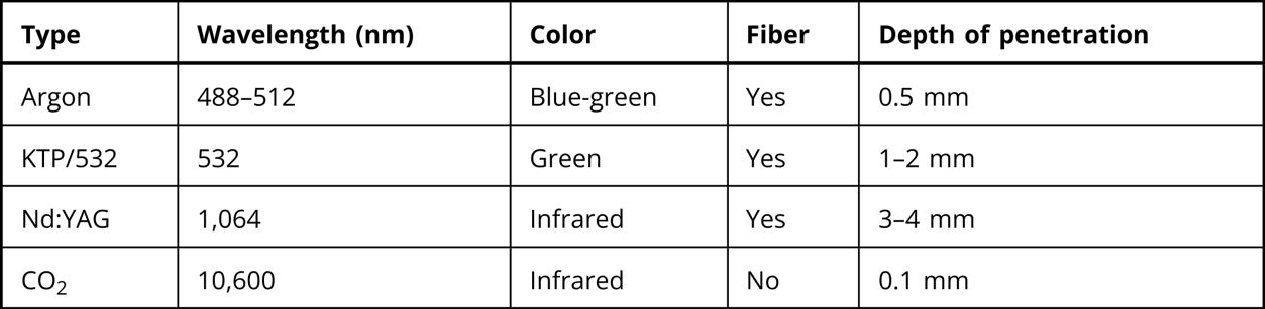
Lasers are named according to the medium that is activated. The common lasers in gynaecology are CO2, argon, KTP, and Nd:YAG. Each medium produces light waves of specific wavelength giving it a characteristic colour (monochromatic). A simple way to understand how light is emitted is to look at an atom with its surrounding electrons (Fig. 1). These electrons occupy discrete orbits that shift to higher orbits when they absorb energy (Fig. 2). Whenever the medium is activated, electrons are displaced to higher energy orbits. In the case of the CO2 laser, activation of the gas particles is done by using electrical wall current. The electrons that are displaced quickly return to their resting orbits, releasing a package of energy in the process referred to as a photon (Fig. 3). This process of light generation is known as spontaneous emission. An example of this process is a light bulb, which emits light waves of different frequencies in all directions out of phase.
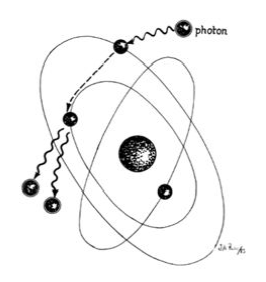
In the laser, however, these photons can further stimulate an already excited atom in its path to release an identical photon that is in phase (coherent), has the same wavelength and colour (monochromatic), and travels in the same direction without divergence (collimated). This process is referred to as stimulated emission (Fig. 4).
Different colours are produced by different lasers. The argon laser produces a wavelength of 510 nm, making a blue-green light. The KTP produces a wavelength of 532 nm, making its light a green colour. The CO2 laser, in contrast, produces a wavelength of 10,800 nm, which is in the nonvisible part of the electromagnetic spectrum, therefore, a helium–neon laser is simultaneously used, which produces a red light to identify the location of the CO2 beam. The laser beam comes out of the port as an unfocused beam. A lens system is used to focus the laser to a focal point. With the hand-held attachment, the focal point is usually ten cm away from the focusing lens. At laparoscopy, the focal length is longer, taking into account the length of the laparoscope. Fine focusing can be done through a joystick or automatically with a coupler. Also, with the use of wave guides, one eliminates the problem of intermittent focusing of the beam that is associated with the joystick device. In contrast to the CO2 laser which is transmitted through long tubes and is reflected by mirrors, the argon, KTP, Nd:YAG and diode lasers are transmitted via a fibre (Figure 5).
Laser Tissue Interaction
There are three basic parameters that determine the amount of energy being delivered to the tissue. The first is the wattage. For most gynecologic procedures using the CO2 laser, one rarely exceeds 20–30 W, which is used primarily for excision purposes. The second parameter is time. The longer the laser remains focused on one spot, the more energy is applied to that area. To limit tissue damage, especially in critical areas, one can simply move the beam back and forth, or select an intermittent, timed pulse mode, usually in fractions of a second. The third parameter that can be controlled is the spot size of the beam. As one gets closer to the target area, the spot size is made smaller, producing a more intense effect. The combination of watts (power) and spot size determines the rate of tissue interaction. The higher the power density, the greater the laser’s ability to vaporize and cut. This concept is expressed in watts/cm2 (unit/area) and referred to as intensity or power density. Power density is, therefore, inversely proportional to the area of the spot size and to the beam diameter. Doubling the beam diameter reduces the power density to one fourth. Conversely, by decreasing the diameter of the spot size, the power density is increased by 4. Of the various lasers available, the CO2 laser remains the most versatile and is relatively safe because of limited depth penetration. The CO2 beam is readily absorbed by tissue because of the tissue’s high-water content. The instantaneous boiling of intracellular water causes cells to explode, forming steam. Depending on the power density, the CO2 laser can be used effectively for vaporizing tissue, for excision, or for incision. Bleeding is reduced with the use of the CO2 laser because of its coagulating properties: it seals small vessels as it cuts. When compared with other lasers, the depth of penetration and the lateral thermal damage of the CO2 laser are limited to less than 1 mm; thus, it can be used in areas of endometriosis on the pelvic side wall near the ureter. In contrast, the Nd:YAG laser has deeper penetration; thus, more caution is needed with its use (Table 1).
Three types of tissue injury may be identified following a laser wound. The zone of vaporization is characterized by an absence of tissue and a V-shaped defect, because energy is greater in the center of the beam than on the perimeter. Immediately below the zone of vaporization is a fixed zone of stromal necrosis measuring (in cervical tissue) approximately 50–100 μm in depth, regardless of the crater’s extent. Within this zone, small vessels (<1 mm) are sealed. The third zone is one of reversible injury or potential repair. Laser wounds are clean, produce minimal tissue damage, and cannot be compared with cautery wounds, in which substantial devitalized debris remains behind. Therefore, laser and scalpel wounds are similar. The CO2 laser may serve three principal functions: as a cutting or excisional instrument, as a vaporizing or ablating instrument, and as a defocused cauterizing instrument.
CO2 Laser
Of the various lasers available, the CO2 laser is the most versatile and is extremely safe because of its limited depth of penetration (0.1–0.5 mm) and lateral thermal damage (0.5 mm). This allows use of the CO2 laser in delicate areas where cautery would be unsafe, such as the bladder, lateral side wall near the ureter, and bowel serosa. Besides vaporization, the CO2 laser. can be used for excision or incision by increasing the power density. Disadvantages of the CO2 laser include focusing of the helium–neon beam as well as production of smoke referred to as “plume,” which needs frequent evacuation to allow adequate visualization of the target.
KTP- Argon Lasers
The KTP and argon lasers have similar wavelengths, 532 nm and 514 nm, respectively, and are delivered via a fibreoptic fibre. They produce an intense green- blue light and can be transmitted through fibers of different diameter (400 μm and 600 μm), thus changing the spot size. The advantages of these lasers over the CO2 laser include: a selective absorption by haemoglobin, less plume production, and an easy delivery system that uses lower power settings in the range of 5–10 W. The main disadvantage is the need to wear special glasses that distort the view of the pelvis and make it difficult to visualize small implants of endometriosis. Keye and colleagues have reported pregnancy rates following argon laser treatment for mild, moderate, and severe endometriosis to be 38%, 30%, and 20%.7
Nd:YAG Laser
Nd:YAG lasers emit an invisible beam with a wavelength of 1064 nm and have to be guided similarly to the CO2 beam using a helium-neon spot. The Nd:YAG wave is readily absorbed by tissue with a deep penetration of 3–4 mm. The energy emitted by the Nd:YAG laser is poorly absorbed by fluids and thus makes it an excellent tool for hysteroscopic surgery. In addition, the beam can be transmitted through an operating hysteroscope. There are two modes of delivery of the Nd:YAG laser: the bare quartz fibre and the quartz fibre that has a sapphire contact tip. The bare fibre on contact with tissue creates an area of coagulation that can extend 3–5 mm into the tissue, as well as peripherally. By using a sapphire tip at the end of the fibre, the laser energy can be focused and converted into heat. This results in the ability to vaporize without the extensive tissue coagulation caused by the bare fibre. Sapphire tips need to be cooled with a coaxial flow of gas or liquid through the fibre and are contraindicated for hysteroscopic surgery, but they can be safely used for abdominal surgery. Recently, it has been made possible to modify bare fibers for use in a contact mode by moulding the tip and creating sculptured tips of various types such as scalpel, tips, and balls. These fibers do not need to be cooled.
Laser safety
Laser surgery in gynaecology has been used for 20 years with a good safety record. However, as with any device used in surgery, a laser has the potential to cause serious injuries. Gynaecologists requesting laser privileges should be certified for the specific type of laser used. Certification implies attendance of didactic instruction and practical use of the laser in the laboratory prior to its application in patients. When using the laser, an appropriate warning sign, such as “Laser in Use,” should be displayed on all doors of the operating room. Protective safety glasses appropriate for the laser in use should always be worn by surgeons and operating room personnel. When the laser is not being fired, it should always be on stand-by mode. Surgical drapes near the operating field have to be fire retardant and kept wet if possible. Adequate suction should be available to collect all plume produced by laser use, because intact viral DNA and papilloma-virus have been detected in the plume (8). When using various types of laser wavelengths, it is important to understand their specific tissue interaction to avoid undesired trauma. It is much easier to cause damage to a vessel or ureter when using deep penetrating energy such as that produced by the Nd:YAG laser than when using the CO2 laser energy, if one is treating superficial endometriosis. In addition, fibers used for transmission of laser energy are delicate and can break. If one is unaware of a broken fibre, laser energy will be delivered at the point of breakage, potentially injuring the patient and/or staff.
Extraperitoneal Laser Surgery
Cervix–Cervical Intraepithelial Neoplasia
Although treatment of cervical intraepithelial neoplasia (CIN) is now more commonly performed. Treatment of CIN can be performed by vaporization or by excisional conization using the laser as a substitute for the scalpel (9). The carbon dioxide (CO2) laser is the laser of choice for this. The major advantages of the CO laser for the treatment of CIN include:
- High degree of clinical efficacy
- Bloodless field
- Microscopic precision
- Sparing of normal tissue
- Rapid healing with minimal scar formation
- Small number of complications
- Outpatient methodology
The main disadvantages of the CO laser include the absence of a histologic sample when vaporization is performed and the expense of the laser machine.
Vaporization
Cervical erosions
When using the laser coupled to the colposcope, one should first define the extent of the lesion. One should keep in mind that endocervical glands may lie deep in the stroma to a depth of 6–7 mm; therefore, treatment should be carried out to a minimum of 9–10 mm with a peripheral margin of 3 mm. This procedure is performed with 30–40 W of power with a 2-mm-diameter spot and takes about 5–10 minutes to complete under local anesthesia (10). Vaporization is performed to a minimal depth of 1 cm and ends at the level of the endocervical canal. The cervical defect should resemble a funnel, as if one performed a small cone biopsy. The operative site is circumferentially outlined with a 3- to 5-mm margin around the lesion. The cervix is then divided into four quadrants. Power is increased to 30–40 W. Beginning in the lower quadrants and using a circular pattern, vaporization is carried down to a depth of 1 cm. The endocervical canal is usually spared. Measurements are made at frequent intervals, relating the depth to the surrounding ectocervical surface. When the lower half of the cervix has been vaporized, a similar procedure is followed for the anterior surface.
The results of CO2 laser treatment of intraepithelial neoplasia are very acceptable and comparable to loop excision. Baggish, Dorsey, and Adelson reported a series of 954 laser excisional cones with 97% showing no evidence of persistent recurrent disease (10). Four cases of invasive cancer were identified, and 73 women had disease extending to the margin; 44 of the latter were followed with no further treatment and remained free of disease. There were 25 women in this series with persistent disease requiring repeat cone or hysterectomy. Complication rates with laser cones are very low; cervical stenosis occurs in 1.3% of cases, cervical incompetence in 0.05%, and major bleeding in less than 1% of patients (11,12).
Vulvar Intraepithelial Neoplasia
Treatment of VIN in the past has included simple vulvectomy and skinning vulvectomy. These more aggressive and disfiguring procedures were replaced by wide local excision and laser ablative procedures of the affected vulvar area (13). Although the CO2 laser has been used widely for ablation of VIN, it does not allow tissue specimen for determination of possible existing invasive disease and identification of margins. Instead of performing laser ablation in treating VIN, the surgeon can obtain laser thin sections (14,15). This method allows the intradermal removal of strips of vulvar skin affected by VIN and at the same time provides a tissue sample for the pathologist to inspect depth of penetration as well as the adequacy of surgical margins (16). With laser ablation for treatment of VIN, cure rates of approximately 80% can be expected (17,18,19). With thin section, these cure rates may exceed 90%. Careful long-term follow-up is imperative. Because vulvar carcinoma in situ is a disease predominantly of younger women, conservative methods of treatment that preserve functional anatomy should be offered to every patient. Even with extensive lesions, laser therapy compares favourably with other methods of treatment.
Condyloma Acuminata
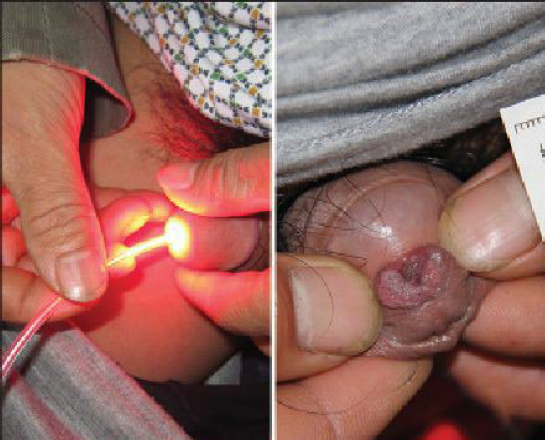
The most common indication for CO2 laser surgery in the lower genital tract is the treatment of condyloma acuminata (20). These lesions caused by the human papilloma virus (HPV) are most commonly found on the vulva, urethra, and perianal skin. Although genital warts can cause irritation and some discomfort, they are not generally painful; most patients seek medical attention because of their unsightly growth. They spread principally by autoinoculation of the HPV virus and may undergo spontaneous regression. The indication for CO2 laser ablation of genital warts is the presence of gross disease. Local anaesthesia may be used for small isolated vulvar warts. However, with more extensive disease, general anaesthesia is preferred (21).
Warts should be vaporized no deeper than the level of the surrounding skin surface (Fig. 6) using a power level of 40– 60 W with a beam diameter of 3 mm. After complete vaporization of the genital warts is accomplished, the 1–2 cm of skin surrounding the individual lesions is brushed lightly with 10 W of power and a 2- mm diameter spot size to destroy subclinical HPV involvement and diminish the recurrence of the disease (22). Laser treatment of genital warts is effective; they are eliminated in over 90% of patients. For those patients who are immunologically compromised, chronic application of 5-fluorouracil cream has been recommended (23,24).
Clinical applications of Laser in Intra-abdominal Gynecological surgery
Carbon dioxide lasers were first used by Bellina and associates in 1974 for intra- abdominal applications (25,26). With improvement in technology and increased experience performing laparoscopic procedures, the use of the laser for laparotomy has been replaced by an endoscopic approach.
Endometriosis
Areas of endometriosis can be vaporized or excised with the CO2 laser depending on the depth of penetration of the lesion. Superficial areas of endometriosis in the cul-de-sac, uterosacral areas, or peritoneum in general can be vaporized with wattages of 10–20 W with spot sizes of 1 mm. With lesions larger than 5 mm, it is recommended that the surgeon cut through a healthy margin of the peritoneum and totally excise the lesion. This scalpel effect can also be accomplished with flexible laser fibers, as well as with electrosurgery and scissors. In addition, the wavelengths of the argon and KTP lasers have an affinity for pigmented lesions such as endometriosis, and these lasers have been used extensively and effectively by Keye and Dixon to heat and vaporize these lesions (27). The CO2 laser, however, remains the most commonly used laser and is the method of choice for treating American Fertility Society (AFS) stage I to IV endometriosis. The main reason is its high margin of safety, which is due to its limited peripheral tissue injury and penetration. This makes it ideal for adhesion excision near ureters and bowel. Although the CO2 laser is ideal for surgical treatment of endometriosis, it does not offer an increased pregnancy rate over other options, including sharp dissection with scissors or electrosurgery (28). Reported pregnancy rates after laser treatment of endometriosis are 57%, 53%, and 61% for mild, moderate, and severe disease, respectively (29,30). Initially, a small opening on the cyst wall is made to allow for aspiration of the chocolate contents and profuse irrigation with a 5-mm aspirator probe. With the CO2 laser used intermittently at 10– 20 W, and with traction and countertraction of the forceps holding the ovarian cortex and endometrium cyst wall, the cyst can be stripped off. The base of the ovary after removal of the cyst wall can then be ablated by defocusing the beam and decreasing to 10 W of energy. This causes coagulation of small areas of oozing and destroys any small areas of cyst wall that may remain (31). The CO2 laser is used to vaporize a lesion 1 cm in diameter by 1 cm in depth at the junction of the uterosacral ligaments to the posterior cervix. A comparison between the CO2 laser and electrosurgery for treatment of dysmenorrhea revealed a 50% relief of pain from both procedures (32).
Adhesiolysis
Pelvic adhesions are common sequelae to previous pelvic infections and pelvic surgery. Although Adhesiolysis is commonly performed with scissors or electrosurgery, the laser is another option and represents an ideal instrument for lysis of vascular adhesions, because excision and coagulation can be performed simultaneously while limiting peripheral tissue trauma. The use of graspers to apply tension on the adhesions maximizes the efficiency of the laser. The use of quartz rods, rods with backstops, and irrigation is recommended to avoid injury to adjacent normal tissue. The pregnancy rate outcome with the laser, however, does not significantly differ from that with other standard modalities used to treat adhesions (33).
Salpingostomy
Correction of distal tubal obstruction by salpingostomy was first reported in 1884 by Schroeder (34). Because of the dismal pregnancy rate, this procedure did not become popular until principles of microsurgery were emphasized. Despite improvements in surgical technique, pregnancy results remained low and dependent on the extent of tubal disease at the time of surgery. Laser salpingostomy is performed, with pregnancy rates similar to those with the laparotomy approach. Treatment of distal tubal blockage begins with lysis of peri tubal and ovarian adhesions until the fallopian tube is completely free and movable. The obstructed end of the tube can be assessed for diameter, presence or absence of fimbriae, and patency. With the CO2 laser at 30 W, two linear incisions in the form of a cross are made in the distal obstructed end of the tube. The edges can then be everted by defocusing the beam on the serosa edges and lowering the energy to 5–10 W (35,36). Continuous irrigation is applied during the procedure. The pregnancy rate with the laparoscopic laser technique is not superior to that with the standard microsurgery technique, and patients remain at risk for ectopic pregnancy in contrast to the low pregnancy rate with neo salpingostomy, fimbrioplasty offers much better results in the 30–60% range because of the limited mucosal damage in these patients. In performing fimbrioplasty, the CO2 laser is used in a continuous mode with 15–20 W of energy, with a backstop and irrigation used as needed. With a spot size of 0.5 mm, the adhesions are easily removed by vaporization (37).
Ectopic Pregnancy
The CO2 laser offers another alternative to electrosurgery and scissors in the treatment of ectopic pregnancy by laparoscopy (38). A linear incision is made on the antimesenteric side of the tube over the dilated portion of the ectopic pregnancy using 30 W of energy with a finely focused beam. A dilute concentration of vasopressin can be injected into the mesosalpinx below the ectopic tissue and on the serosa overlying the ectopic pregnancy to decrease intraoperative bleeding (39). With the use of hydro dissection, the ectopic tissue is then gently lifted from its tubal bed. Small amounts of vascular oozing can be controlled with the laser, or alternatively with bipolar forceps (40,41).
Uterine Fibroids
Uterine leiomyomas are extremely common benign smooth muscle tumours occurring in 25% of women older than 30 years of age (42,43). Laser energy can be delivered through a handpiece for laparotomies or via the laparoscope. Small fibroids can be vaporized directly with 20–30 W of energy or can be shelled out in their entirety, as during conventional surgery. Although the laser is capable of coagulation, it is necessary to suture or coagulate larger vessels (greater than 0.5 mm) with electrical energy preferably bipolar. The advantages of the CO2 laser for removal of uterine broids over scissors and standard electrosurgery are improved haemostasis, decreased tissue trauma, and decreased severity of adhesion formation. Approximately 50–60% of patients ultimately achieve pregnancy with laser myomectomy (44).
Ovarian Wedge Resection
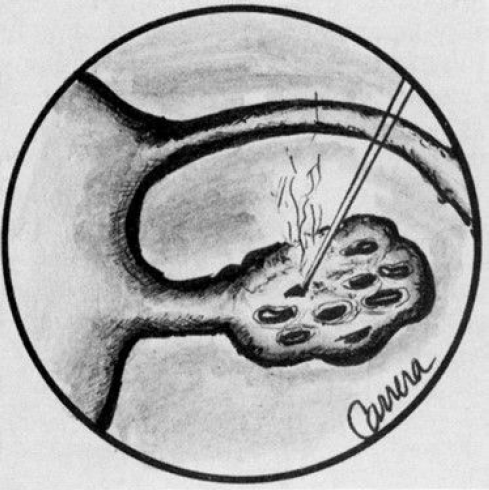
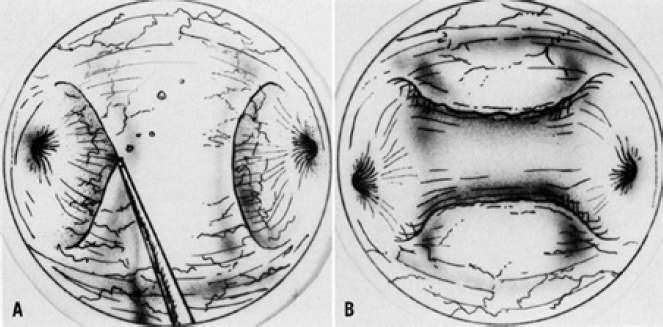
Ovarian wedge resection by laparotomy, once a popular treatment option for patients resistant to clomiphene citrate, is now rarely performed because of the development of severe postoperative adhesions. However, with strict adherence to microsurgical technique and the use of ne electrocautery needle tips or lasers, it is possible to perform ovarian wedge resection with pregnancy rates of 40%, which may be due to less adhesion formation (45). Once the ovaries are exposed, they are draped with moist sponges. With 30 W of energy, a focused beam of 0.5 mm can be used as a scalpel to remove a wedge of the ovary. To achieve the same endocrinologic effect of lower ovarian androgen levels and ovulation that occurs with laparotomy, laparoscopic electrocoagulation or laser coagulation of the ovary can be performed (46). With this technique, either each ovary is cauterized with a unipolar needle electrode, or laser energy is applied in multiple areas, causing small ovarian craters. (Fig- 7) The CO2, KTP, argon, and Nd:YAG lasers have been used to treat polycystic ovaries with ovulation rates of 70% and pregnancy rates of 40% (45).
Laser Hysteroscopy
The hysteroscope has been used for many years as a diagnostic instrument to evaluate the source of abnormal uterine bleeding. With refinement of light sources, the use of low-viscosity fluids, and newer operating hysteroscopes, it is now possible to use this technology as a therapeutic modality for patients with abnormal uterine bleeding. Various instruments can be used with the hysteroscope, including electrodes to cut and coagulate, operating graspers, scissors, as well as various laser fibers. The main laser delivery systems available for hysteroscopy are the Nd:YAG and KTP 532. Both of these lasers use a flexible fibre that can be passed easily through the operating sleeve of the hysteroscope.
Endometrial Ablation
Although there are many indications for hysterectomy, dysfunctional uterine bleeding is the indication given in as many as half of these procedures when there is no organic cause (48,49).
The purpose of an endometrial ablation is to destroy the entire endometrium and avoid future regeneration and menstrual bleeding. Patients are initially pretreated with a gonadotropin releasing hormone (GnRH) agonist or danazol for a period of four weeks to decrease the endometrial thickness to that seen in the menopausal state, thus facilitating the penetration of the energy to the level of the myometrium.
After adequate visualization of the entire uterine cavity, the laser fibre can be inserted through the operating channel of the hysteroscope. The Nd:YAG fibre can be used as either a touch or no touch technique. With the touch technique, the laser fibre is activated with 40–50 W of energy and dragged on the endometrial surface beginning on the fundus and traveling down toward the endocervix in successive strokes (Fig- 8). This is done in a systematic way so that the entire surface is eventually covered. With the no touch or blanching technique, the laser fibre is placed a few millimetres away from the endometrial surface while the laser energy is activated. Of the two methods, the touch technique is preferred because penetration is deeper, extending 4–6 mm into the uterine wall. This depth is sufficient for destruction of the endometrium (50).
Septate Uterus
The uterine septum is one of several congenital uterine abnormalities that arise from incomplete resorption of the Müllerian ducts, and it occurs in approximately 1–3.5% of women (51). The uterine septum is commonly associated with habitual miscarriage and is transmitted as a polygenic or multifactorial pattern of inheritance. Resection of a uterine septum can be performed with various instruments, including scissors, resectoscope, and Nd:YAG laser. The Nd:YAG laser fibre is usually set at 40 W and used by the touch technique, as if one were using a scalpel. The laser tip must be oriented so that it incises the septum at the midline and does not deviate from this line of incision (Fig. 9). This will ensure a relatively bloodless eld of incision and avoids injury to the myometrium. The laser incision is continued until there is uniformity in light transmission throughout the fundus as observed by laparoscopy, or until bleeding from the fundal myometrium is visualized. The advantage of the laser fibre technique over the scissor technique is primarily one of diminished bleeding. The procedure usually takes 20–30 minutes, with gratifying results such as a 70–80% delivery rates.
Other clinical applications of lasers in gynecology and reproductive medicine
Laser treatment for genitourinary syndrome of menopause (GSM)
GSM is a chronic condition affecting approximately 40 %–60 % of postmenopausal women (52). The first line treatment is low dose vaginal oestrogens while vaginal moisturisers or oral ospemifene are the alternative options. Non-hormonal options such as intravaginal laser have potential when vaginal oestrogens are contraindicated or ineffective and for women who decline hormones (53). The safety of vaginal fractional CO2 laser therapy has been shown in several randomised controlled trials (RCTs)). The efficacy of the CO2 laser was found to be similar to vaginal oestrogens for GSM symptoms (54). However, RCTs comparing the micro ablative CO2 laser with medical treatment have reported conflicting results. Salvatore et al. and Ruanphoo et al demonstrated the superiority of the laser while Li et al. and Page et al. showed that the treatment response after laser application was comparable to that of sham application (55 – 58).
Laser for vaginal laxity and pelvic organ prolapse (POP)
Vaginal laxity is a poorly understood but common symptom of pelvic floor dysfunction that currently lacks a standardised definition. It may be considered a symptom of prolapse and it is a manifestation of levator ani hyper distensibility (59). The use of laser therapy for vaginal laxity and POP is still a relatively new approach, with limited available evidence for its efficacy and safety (60). Supervised pelvic floor muscle training and use of pessaries are established non-surgical options for treatment of POP. Ogrinc et al. evaluated the effects of non-ablative Nd:Yag in 61 women with a stage 2 to 4 cystocele (61). Follow-up visits were performed at 2, 6 and 12 months. The authors report a consistently significant anatomical improvement throughout the study period. Of note, a control group was not evaluated. Athanasiou et al. enrolled 30 postmenopausal women, who were awaiting surgery for a symptomatic stage 2/3 cystocele and/or rectocele (62). These were randomised to either non-ablative ND:YAG laser treatment or watchful waiting. A stage 0 or 1 POP (“objective cure”) at 4 months following laser treatment was primarily evaluated. However, none of the patients were cured. In the laser group, the POP stage remained unchanged in 11/15 (74 %) of participants and decreased by one stage in 2/15 (13 %). There are no good quality studies to evaluate the use of laser for women with vaginal laxity.
Laser treatment for stress urinary incontinence (SUI)
The first line treatment for SUI is supervised pelvic floor muscle training. Due to the concerns about the use of synthetic mid urethral slings, vaginal lasers have been promoted as a potential treatment option for SUI. Recently, four RCTs have been published that have shown conflicting results. Regarding the ND:YAG laser, O’Reilly et al. conducted a multicentre sham-controlled trial including 110 women with urodynamic SUI (63). A standardised one-hour pad weight test was performed at baseline and at 6-month follow-up. A greater than 50 % reduction in the pad weight was considered as primary outcome. Of 89 women followed-up, treatment success was observed in 33/56 (59 %) in the active arm and 12/33 (36 %) in the non-treated arm. The authors conclude that women treated with ND: YAG laser had a three-fold higher chance of success, with an odds ratio of 3.6 (95 % CI: 1.3 – 11.2, p-value =0.02). Interestingly, women with mild to moderate SUI appeared to have benefitted from the laser treatment to a greater degree compared to women with severe SUI. These findings contrast with the results reported by a single site RCT from Canada which enrolled 134 women with a clinical diagnosis of SUI (64). Over 90 % of participants from either ND:YAG laser (67/73) and control (58/61) treatment were followed- up at 6 months. A self-reported symptom of no urinary incontinence with the International Consultation on Incontinence Questionnaire- Urinary Incontinence (ICIQ-UI) Short Form (SF) was evaluated as primary outcome. “Cure” was reported by one patient only in each group. Both laser and control groups showed an improvement in ICIQ-SF total scores at 6-months, but there was no significant difference in the changes from baseline between groups. There are also conflicting reports for the CO2 laser. A single centre RCT from Seki et al evaluated the subjective impression of improvement in SUI (Likert scale) an objective cure as primary outcomes (64). At 12-month follow-up, both outcomes were significantly better in the laser (n =38) when compared to the control (n =38) group.
Lasers in Reproductive medicine- Laser Assisted Hatching
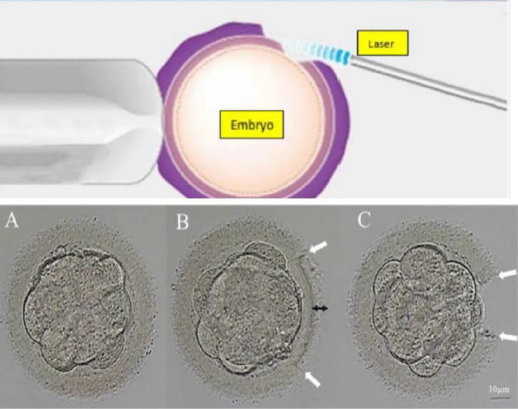
Although ART has achieved a 30–50% average pregnancy rate, the embryo implantation rate (IR) remains low, at about 20–30%. Implantation, an extremely complicated biological process that consists of embryo hatching, localization, attachment, and invasion, is influenced by many factors. Hatching of a blastocyst is a critical step in the sequence of physiologic events culminating in implantation. Failure to hatch due to intrinsic abnormalities in either the blastocyst or the zona pellucida (ZP)may be one of many factors limiting human reproductive efficiency.
Assisted hatching (AH) involves artificial thinning or breaching of the ZP and has been proposed as one technique to improve implantation and pregnancy rates after in vitro fertilization (IVF). An increased implantation rate after mechanical opening of the ZP (partial zona dissection) was first reported in 1990. Lasers, chemical agents, and mechanical methods are often used to achieve ZP destruction, including thinning, drilling, and full-thickness removal (65). Laser-assisted hatching (LAH) first emerged in the early 1990s. Due to its accuracy, speed, and safety, LAH is the most common method. The use of AH is prevalent in clinical practice, accounting for nearly 44.8% of in vitro fertilization (IVF) cycles in the USA between 2000 and 2010. Historically, assisted hatching was performed before embryo transfer on days 3, 5, or 6 after fertilization using various methods, including creation of an opening in the ZP by thinning with acidified Ty- rode solution , partial ZP dissection with a glass microneedle, laser photoablation, or use of a piezo micromanipulator. Currently, AH is most commonly performed with full-thickness, laser-AH on the day of embryo transfer. Types of lasers used for ablating zona pellucida;
- Nd:YAG laser at 1064 or 534nm
- Tuneable Titanium: sapphire lasers at 650– 1080nm
- Semiconductor laser emitting at 1.48 mm, which is the highest standard for laser ART fulfilling all safety requirements for zona pellucida ablation.
Indications of assisted hatching
- Age of the women is older than 37 years
- Woman with poor ovarian reserve: low AMH levels/ low AFC (antral follicle count)/ high levels of follicular stimulating hormone on day 2/3 of periods.
- Women with poor-quality embryos. (excessive fragmentation or slow rate of cell division)
- Zona factor-cases with thick outer shell (zona pellucida) 5. One or more previous failed IVF cycles
Laser Assisted hatching techniques
Zona pellucida thinning and Zona pellucida drilling. Zona pellucida can be thinned for one quarter or half of the total circumference. Zona pellucida can be drilled with holes <10mm- >25mm diameter. Complications of Laser hatching;
- damage to the embryo
- damage to individual blastomeres with reduction of embryo viability.
- Monozygotic twinning
Laser acupuncture in Female Infertility
Needle and laser acupuncture are forms of complementary alternative medicine that are effective for treating a variety of conditions, such as chronic pain, wounds, inflammation, and depression. These treatments have also been used to improve the pregnancy rate. Infertility treatments increase psychologic stress, and several reports have indicated that acupuncture treatment alleviates emotional distress in infertile women (66). IVF-ET and ICSI involve a number of stressful aspects: daily injections; blood sampling; ultrasounds; laparoscopic surgery; and the possibility of failure at any of the various stages. The stress experienced by infertile women changes over time. In the early stages of infertility, most of the stress these women experience derives from a physical inferiority complex, while, later on, they tend to become stressed about what others outside of their families will say. It has been also found that infertile women had persistently elevated stress and anxiety levels during IVF-ET.
Laser acupuncture activates the inferior parietal lobule, the primary somatosensory cortex, the praecuneus of the parietal lobe, and the medial and superior frontal gyri of the frontal lobe, which is associated with mood changes. Few studies show the use of laser and needle acupunctures in ameliorating emotional distress in women undergoing infertility treatments. This intervention before and after embryo transfer has resulted in higher implantation rates and live birth rates.
Lasers in Male Infertility (Low Level Laser Therapy)
Phototherapy with a low-level laser is referred to as photo- biomodulation. Motility is one of the most prominent characteristics of sperm associated with the fertilizing capability. The mitochondrial apparatus within the midpiece of the spermatozoa provides the required energy for movement of the flagellum or tail. Low- level laser irradiation induces the activity of the cytochrome c oxidase (COX). The COX complex is part of the mitochondrial respiratory chain and plays a critical role in the electron transport cascade. Modulation of this certain cytochrome oxidase activity leads to enhanced oxidative phosphorylation or adenosine triphosphate (ATP) generation. This process would subsequently augment the sperm motility. On the molecular level, the LLLT is mediated in the up- regulation of genes coding for a number of mitochondrial enzymes. Specifically, the subunits which are involved in the complexes I and IV of electron transport chain and ATP synthase. Phototherapy also increases ATP synthesis in the myotubes (67). It interacts with the endogenous cellular redox mechanisms. This effect is mediated through photoexcitation of cytochrome c oxidase in the mitochondrial electron transport chain. Laser light facilitates electron transferring to the oxygen molecules and production of the reactive oxygen species (ROS). These anions are categorized into three main types, namely superoxide, hydrogen peroxide, and hydroxyl radical. ROS are necessary for the process of spermatozoa maturation or capacitation. Low levels of reactive oxygen species (ROS) relatively enhance the sperm acrosome reaction. Photo-biomodulation resulted in a significant increase in the human sperm motility and capacitation toward activation of protein kinase A and sarcoma protein kinase, as well as the production of reactive oxygen species. Another aspect of photo-biomodulation is the effect of irradiation on the intracellular calcium ion levels, which has a fundamental impact on the sperm motility. Low-level laser therapy increases the calcium influx by means of cellular pumps. In this regard, Na+/Ca2+exchanger and voltage-gated calcium channel regulate the optimal intracellular calcium concentrations. LLLT prevents the calcium uptake by mitochondria of spermatozoa while enhancing the Ca2+ binding to sperm plasma membrane. On the other hand, laser light at higher doses causes an overload in the intracellular Ca2+ levels. Such a process leads to hyperactivation of the Ca2+-ATPase pump and exhausts the ATP reservoir of the cells. These particular reactions would ultimately increase the intracellular osmotic pressure and degenerate the spermatozoa. Effect of the laser therapy on the sperm parameters is directly related to the semen sample quality, irradiation methods, applied doses, wavelengths, and time intervals.
This fact emphasizes the importance of selecting the optimal output power Human sperm motility as well as velocity can be improved by Helium-Neon laser irradiation. It was found that irradiation of human sperm with broad band visible light (400–800 nm) caused a significant increase in hyperactivated
Lasers in Male Infertility (Low Level Laser Therapy)
Phototherapy with a low-level laser is referred to as photo- biomodulation. Motility is one of the most prominent characteristics of sperm associated with the fertilizing capability. The mitochondrial apparatus within the midpiece of the spermatozoa provides the required energy for movement of the flagellum or tail. Low- level laser irradiation induces the activity of the cytochrome c oxidase (COX). The COX complex is part of the mitochondrial respiratory chain and plays a critical role in the electron transport cascade. Modulation of this certain cytochrome oxidase activity leads to enhanced oxidative phosphorylation or adenosine triphosphate (ATP) generation. This process would subsequently augment the sperm motility. On the molecular level, the LLLT is mediated in the up- regulation of genes coding for a number of mitochondrial enzymes. Specifically, the subunits which are involved in the complexes I and IV of electron transport chain and ATP synthase. Phototherapy also increases ATP synthesis in the myotubes (67). It interacts with the endogenous cellular redox mechanisms. This effect is mediated through photoexcitation of cytochrome c oxidase in the mitochondrial electron transport chain. Laser light facilitates electron transferring to the oxygen molecules and production of the reactive oxygen species (ROS). These anions are categorized into three main types, namely superoxide, hydrogen peroxide, and hydroxyl radical. ROS are necessary for the process of spermatozoa maturation or capacitation. Low levels of reactive oxygen species (ROS) relatively enhance the sperm acrosome reaction. Photo-biomodulation resulted in a significant increase in the human sperm motility and capacitation toward activation of protein kinase A and sarcoma protein kinase, as well as the production of reactive oxygen species. Another aspect of photo-biomodulation is the effect of irradiation on the intracellular calcium ion levels, which has a fundamental impact on the sperm motility. Low-level laser therapy increases the calcium influx by means of cellular pumps. In this regard, Na+/Ca2+exchanger and voltage-gated calcium channel regulate the optimal intracellular calcium concentrations. LLLT prevents the calcium uptake by mitochondria of spermatozoa while enhancing the Ca2+ binding to sperm plasma membrane. On the other hand, laser light at higher doses causes an overload in the intracellular Ca2+ levels. Such a process leads to hyperactivation of the Ca2+-ATPase pump and exhausts the ATP reservoir of the cells. These particular reactions would ultimately increase the intracellular osmotic pressure and degenerate the spermatozoa. Effect of the laser therapy on the sperm parameters is directly related to the semen sample quality, irradiation methods, applied doses, wavelengths, and time intervals.
This fact emphasizes the importance of selecting the optimal output power Human sperm motility as well as velocity can be improved by Helium-Neon laser irradiation. It was found that irradiation of human sperm with broad band visible light (400–800 nm) caused a significant increase in hyperactivated motility, but not in total motility, of human sperm. Biochemical and topological analysis evidenced that fertilizing increased in irradiated sperms. Helium-neon irradiation increased the sperm motility index, viability, and cell energy charge. Hence, might be a useful technique for enhancing the quality of semen in long-term storage. Significant increase in sperm motility was observed with irradiation of cells at doses of 4 and 6 J/cm2 at 60 and 45 minutes after irradiation.
Lasers in Cosmetic Gynaecology
Cosmetic gynaecology represents a rapidly advancing subspecialty, wherein the integration of laser technologies has significantly enhanced both the aesthetic and functional outcomes of various procedures. The precise, minimally invasive nature of laser-based interventions has transformed the landscape of cosmetic gynaecology, offering patients superior results with reduced morbidity and shorter recovery periods.
Vaginal Rejuvenation
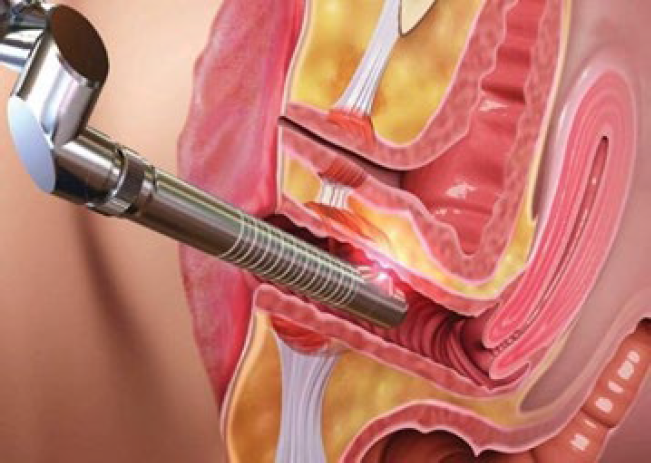
Vaginal rejuvenation, an umbrella term encompassing a spectrum of procedures aimed at enhancing vaginal tightness, improving sexual function, and ameliorating vaginal health, has been revolutionized by the advent of laser technology. The primary modalities employed are fractional CO2 lasers and erbium lasers. These lasers induce controlled thermal injury to the vaginal mucosa and submucosa, leading to nucleogenesis and the remodelling of elastin fibers. The resultant tissue contraction and increased collagen deposition restore vaginal tone and elasticity, mimicking the effects of surgical tightening without the associated invasiveness.
The procedure itself is typically conducted in an outpatient setting, where a specially designed laser probe is introduced into the vaginal canal (Fig. 11). The probe emits laser energy circumferentially, targeting the entirety of the vaginal wall. This non-ablative approach is particularly advantageous in patients with mild to moderate vaginal laxity, those experiencing decreased vaginal lubrication, or individuals with stress urinary incontinence secondary to pelvic floor dysfunction. Post-procedural recovery is expedient, with minimal discomfort, and most patients can resume their routine activities, including sexual intercourse, within a week.
Labial Trimming (Labiaplasty)
Labiaplasty, or labial trimming, focuses on the aesthetic and functional refinement of the labia minora and, less commonly, the labia majora. Lasers have emerged as a superior tool in this context due to their precision, haemostatic properties, and reduced postoperative morbidity. The CO2 laser is particularly favoured for its ability to precisely ablate the redundant labial tissue while simultaneously coagulating blood vessels, thereby minimizing intraoperative blood loss and reducing the risk of hematoma formation.
Patients seeking labiaplasty may present with hypertrophic or asymmetrical labia minora, leading to physical discomfort during activities such as cycling, intercourse, or when wearing tight clothing. Beyond the physical symptoms, there is often a significant psychological component, with patients experiencing distress related to the appearance of their genitalia. The laser technique not only allows for the precise excision of excess tissue but also facilitates the sculpting of the labial edges to achieve a more aesthetically pleasing contour. The reduction in thermal spread afforded by laser technology also contributes to faster wound healing and reduced scar formation, enhancing the overall cosmetic outcome. Postoperative recovery typically involves a few days of swelling and discomfort, with complete resolution within a few weeks.
Vulvar Melanosis Treatment
Vulvar melanosis, characterized by benign hyperpigmented macules on the vulvar skin, often presents a cosmetic concern despite its benign nature. Laser treatment has proven to be an effective method for addressing this condition, with Q-switched Nd and fractional CO2 lasers being the most commonly utilized modalities. These lasers specifically target melanin within the pigmented lesions, resulting in selective photothermolysis. The laser energy fragments the melanin granules without damaging the surrounding keratinocytes or dermal structures, allowing for a gradual lightening of the hyperpigmented areas.
The procedure is typically performed under local anaesthesia, with multiple treatment sessions often required to achieve optimal results. Patients can expect significant improvement in the uniformity of the vulvar skin tone, with results being long-lasting. However, maintenance sessions may be necessary depending on the patient’s propensity for pigmentation and external factors such as UV exposure. The post-treatment recovery is generally uncomplicated, with mild erythema or oedema resolving within a few days.
Liposuction of the Pubic Mound (Mons Pubis)
The reduction of the mons pubis, particularly when characterized by excessive adiposity, is another area where laser-assisted techniques have gained popularity. Excess fatty tissue in this region can lead to discomfort, altered body image, and difficulty with clothing fit. Traditional liposuction techniques, while effective, often result in significant tissue trauma and prolonged recovery.
The introduction of laser-assisted liposuction, such as with the SmartLipo system, offers a minimally invasive alternative with enhanced outcomes. In laser-assisted liposuction, a fine laser fibre is introduced through a small incision, delivering targeted laser energy to the adipose tissue. This energy liquefies the fat cells, facilitating their removal via aspiration while simultaneously stimulating collagen synthesis within the overlying dermis. This dual action not only reduces the volume of the mons pubis but also contributes to skin tightening, addressing issues of skin laxity that may accompany fat removal. The procedure is typically performed under local anaesthesia, with patients experiencing minimal discomfort and a swift return to daily activities.
Vulvar Hair Removal
Laser hair removal has become a cornerstone in the management of unwanted vulvar hair, offering a long-term solution with significant advantages over traditional methods such as shaving or waxing. The diode, Nd:YAG, and alexandrite lasers are the primary tools used in this context, each selected based on the patient’s skin type and hair colour.
These lasers operate by emitting light energy that is absorbed by the melanin within the hair follicles, leading to selective photothermolysis. This process destroys the hair follicle while sparing the surrounding tissue, resulting in a substantial reduction in hair density and regrowth over successive treatments. Laser hair removal in the vulvar region typically requires multiple sessions to target all hair follicles during their active growth phase (anagen phase). The result is a marked reduction in hair growth, smoother skin, and a decreased incidence of folliculitis and ingrown hairs. The procedure is generally well-tolerated, with transient erythema and mild discomfort being the most common side effects, both of which resolve quickly.
Complications of Lasers in Gynecology
The application of laser technology in gynaecology has brought significant advancements in minimally invasive surgical techniques, but it also presents a range of potential complications that must be carefully managed. These complications can manifest at various stages—immediate, short-term, or long-term—and require a deep understanding of laser-tissue interactions to minimize risks and ensure patient safety.
Thermal injury is one of the most immediate and significant complications associated with the use of lasers in gynecology. Lasers work by generating heat, which is precisely applied to target tissues for ablation, coagulation, or vaporization. However, when not adequately controlled, this thermal energy can cause unintended damage to adjacent tissues. This can result in superficial burns or deeper tissue necrosis, leading to pain, delayed wound healing, and scarring. The risk of thermal injury is heightened in areas where tissue layers are thin or highly vascular, requiring meticulous technique and precise calibration of the laser settings.
Another immediate concern during laser procedures is intraoperative bleeding, although lasers are often chosen for their ability to coagulate blood vessels effectively. In some cases, improper technique or excessive energy application can result in damage to blood vessels beyond the intended treatment zone, leading to bleeding. This is particularly problematic in areas with rich vascularization, such as the cervix or vaginal wall. Managing such bleeding during the procedure can be challenging and may necessitate additional interventions to control haemorrhage and maintain a clear surgical field.
Smoke plume inhalation is a less obvious but significant risk associated with laser use in gynaecology. The ablation of tissue by lasers generates a plume of smoke that contains not only carbonized particles and chemicals but also potentially infectious agents, such as viral DNA, including that of the human papillomavirus (HPV). Inhalation of this smoke can pose health risks to both the patient and operating room staff, making the use of effective smoke evacuation systems and appropriate personal protective equipment essential to minimize exposure.
In the short-term postoperative period, pain and discomfort are common complaints among patients undergoing laser procedures. The degree of discomfort varies depending on the extent of the tissue ablation and the individual patient’s pain tolerance. This pain is often a result of the inflammatory response triggered by the tissue injury. Effective postoperative pain management, including the use of analgesics and anti-inflammatory medications, is crucial to enhance patient comfort and facilitate recovery.
Infection is another potential short-term complication following laser surgery. While laser procedures generally have a lower risk of infection compared to traditional surgical methods, the disruption of natural tissue barriers and alteration of local flora can still predispose patients to bacterial infections. This is particularly a concern in procedures involving the genital tract, where the natural microbial environment can be disturbed. Preventative measures, including the use of perioperative antibiotics and strict aseptic technique during the procedure, are essential to reduce the risk of postoperative infections. Delayed wound healing and scarring are further concerns, particularly in patients with underlying conditions such as diabetes or those who are immunocompromised. The precise nature of laser ablation means that while tissue damage can be minimal, the healing process may still be prolonged in certain individuals. Scarring can also occur, particularly if the laser energy is not appropriately calibrated or if the tissue response is more pronounced. This is of particular concern in cosmetic Gynecological procedures, where the aesthetic outcome is a critical component of patient satisfaction.
Long-term complications, while less common, can include persistent pain, chronic scarring, and functional impairments depending on the area treated and the extent of tissue involvement. For instance, in cases where deep tissue structures are inadvertently damaged, patients may experience chronic pain or sensitivity in the treated area.
Additionally, in procedures like vaginal rejuvenation or labial trimming, improper technique or excessive tissue removal can result in functional deficits, such as reduced elasticity or altered sensation, which can impact sexual function and overall quality of life.
Conclusion
While lasers have significantly improved the precision and outcomes of gynecological surgeries, they are not without their risks. Complications such as thermal injury, intraoperative bleeding, smoke plume inhalation, postoperative pain, infection, delayed healing, and scarring highlight the importance of careful patient selection, meticulous surgical technique, and thorough postoperative care. Understanding these potential complications allows for better risk management and enhances the overall safety and effectiveness of laser use in gynecology.
New Avenues for research
Customized Laser Parameters
Research can enhance personalized laser settings (wavelength, fluence, pulse duration) for optimized selective photothermolysis, reducing thermal damage, particularly in sensitive gynecologic structures.
Longitudinal Data on Aesthetic and Functional Procedures
Investigating long-term histological and functional outcomes of procedures like fractional CO₂ and Er lasers for GSM and vaginal laxity could validate durability and safety.
Minimally Invasive Laser Ablation for Endometriosis
Precision laser ablation (e.g., KTP, diode lasers) could become a conservative, fertility-preserving alternative for selective endometriotic implant treatment with minimal surrounding tissue impact.
Combination Laser-Adjuvant Modalities
Studying the synergy of laser with PRP or stem cell therapy for conditions like vulvovaginal atrophy and urinary incontinence could validate enhanced regenerative effects.
Robotic-Assisted Laser Surgery
Using robotics with CO₂ or thulium laser optics could refine complex gynaecological surgeries, such as nerve-sparing endometriosis excision or myomectomy, reducing complications and improving outcomes.
Photobiomodulation for Urogynecologic Disorders
Photobiomodulation (PBM) could support pelvic floor restoration by activating fibroblasts and enhancing tissue architecture, particularly for stress urinary incontinence and mild prolapse.
Photodynamic Therapy for Early Gynecologic Neoplasia
PDT with targeted photosensitizers could serve as a non-invasive option for early-stage cervical and vulvar neoplasia, preserving surrounding tissue.
Laser Therapy in Pediatric and Adolescent Gynecology
Trials on laser use for vulvar lichen sclerosus, genital warts, and labial adhesions in young populations are essential to establish safety and efficacy in this unique patient group.
Treatment for Vulvar Dermatoses and Vulvodynia
Lasers could be researched for pain modulation and collagen remodeling in chronic conditions like vulvodynia, lichen planus, and lichen sclerosus, addressing intractable symptoms.
Image-Guided Laser for Precision in Complex Pathologies
Integrating laser systems with real-time imaging (ultrasound/MRI) could enable precise ablation of fibroids and adenomyosis, preserving uterine integrity in challenging pelvic locations.
References
Figure 1: Illustration of an atom with a nucleus orbiting electrons
Figure 2: Stimulated absorption occurs as a photon impacts on an electron, driving it into a higher orbit
Figure 3: Slow, natural decay causes an electron to drop into a lower orbit, resulting in a photon being emitted at a predicted frequency equal to the difference in energy between the two orbits
Figure 4: The natural ‘slow’ decay process may be stimulated by collisional processes, resulting in many photosn traveling at the same frequency as the inducing photons
Figure 5 – Flexible fibers used for laser surgeries.
Table 1: Types of Lasers used in gynaecology
Figure 6- Laser treatment for urethral condyloma acuminate
Figure 7- Ovarian wedge craters produced by CO2 laser
Figure 8- Endometrial ablation with Nd:YAG laser fibre
Figure 9 – Uterine septum treated with Nd:YAG laser
Figure 10: Laser Assisted Embryo Hatching
Figure 11: Vaginal rejuvenation