Authors / metadata
DOI: 10.36205/trocar4.2023002
Abstract
Introduction: Uterine isthmocele is a pouch-like structure defect at the site of a previous cesarean scar. Due to an increase in cesarean rates over the years, prevalence of isthmocele has grown and is estimated to range from 19.4% to 88%. While it may not always cause symptoms, it can lead to disabling symptoms. Various studies have suggested that surgical management can be beneficial in reducing symptoms and improving obstetric outcomes. Nonetheless, there is still conflicting data on which surgical approach is best suited for patients with symptomatic isthmocele. This study aims to evaluate and compare each surgical approach for these patients.
Method: A systematic search was performed in PubMed, ScienceDirect, Embase, and Cochrane from January 2000 until June 2023 to compare surgical approaches outcome for symptomatic isthmocele patients. The research were using MeSH terms if applicable and following the PRISMA guidelines. MedCalc was used to see the overall proportion of each study and Review Manager 5.4 was used to calculate the result of 95% CI for the outcomes. Newcastle–Ottawa scale was used to assess the risk of bias. Primary endpoints of interest were improved symptoms, duration of menstruation post-treatment, and postoperative complications.
Results: Twelve Non-comparative studies and six comparative studies with 1389 patients were included. Pooled analysis of proportion showed an improvement of symptoms in 90.74% (75.02% – 95.19%) after hysteroscopic surgery, 84.33% (52.55% – 93.27%) after laparoscopic surgery, and 65.71% (51.20% – 78.86%) after transvaginal surgery. Interestingly, in the pooled analysis of comparative surgery, laparoscopy significantly resulted in shorter menstruation duration than hysteroscopy MD 2.47, 92% CI (1.83 – 3.11) <0.001, I2: 0%. However, hysteroscopy had better outcomes in terms of operative duration, blood loss, and hospital stay.
Conclusion: This is the initial meta-analysis that compares various surgical options for treating symptomatic isthmocele. Overall, more than 60% of patients experienced an improvement in their symptoms. For those with enough residual myometrial thickness overlapping the isthmocele, hysteroscopic surgery was deemed the safest and most effective option. However, laparoscopy showed greater improvement in menstruation duration compared to hysteroscopy. Patients with thinner residual myometrium may benefit more from laparoscopy or transvaginal surgeries.
Introduction
The cesarean section is a frequently performed gynecological procedure, with its occurrence steadily rising (1). It constitutes one-third of all deliveries globally. The World Health Organization advocates for an optimal cesarean section rate of 10-15% of all births. However, in South America (42.9%), Latin America (40.5%), North America (32.3%), and Europe (25%), the percentage of cesarean section deliveries exceeds this number (2,3). There is currently a global conversation surrounding the risks and outcomes associated with cesarean section births, and these risks are becoming more prevalent. Certain complications, such as scar dehiscence, placenta previa, and accreta, have already been extensively researched. However, other complications, like cesarean scar defects, isthmoceles, and niches, are just beginning to gain attention (4).
Isthmocele or caesarean scar defect (CSD) is a condition with a pouch-like defect in the front wall of the uterus connected to the uterus cavity. It is mainly caused by medical intervention or as an iatrogenic disease such as a cesarean section. The healing process of the uterine wall after the surgery does not restore the myometrial layers properly, leading to a fibrotic reaction resulting in a minor resistance locus (5). This was caused because the uterine isthmus of the myometrial layers after hysterotomy in the anterior wall did not heal appropriately. As the number of previous cesarean sections increases, so does the occurrence of isthmocele, a defect in the cesarean scar. Recent data shows that 60% of patients have an isthmocele after their first cesarean section and 100% after three cesarean sections. Although isthmocele can be asymptomatic, it may cause adverse reproductive outcomes, pelvic pain, and abnormal uterine bleeding. During the menstrual cycle, a small amount of blood can accumulate in a pouch-like defect. This causes an inflammatory reaction and may contribute to irregular vaginal bleeding, pelvic pain (due to the effects of inflammatory cytokines on perilesional nerve fibers), and difficulties with embryo implantation that result in secondary infertility or subfertility. Also, isthmocele may increase the rate of cesarean scar pregnancy, a potentially life-threatening condition that deserves accurate management to avoid massive uterine bleeding (6,7).
Isthmocele can be presented in transvaginal ultrasound (TVUS) or sonohysterography (SHG) examination in non-pregnant women following a previous cesarean scar (CS). The location of the defect varies depending on the location of the CS, the stage of labor, changes in the uterine cervix, and the surgical technique. There are different ways to treat CSD, both surgical and non-surgical. It has been suggested that oral contraceptives, hormonal intrauterine device may help reduce AUB in isthmocele patients. Surgical methods include laparotomic, laparoscopic, and vaginal excision and repair, as well as resectoscopic revision with radiofrequency ablation of the isthmocele base and electrosurgery. The purpose of this review is to evaluate the evidence supporting the surgical treatment of isthmocele in women with abnormal uterine bleeding and infertility, as well as in preventing obstetric complications. Additionally, we will examine the potential risks and complications associated with these surgical treatments.
Material & methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (8). This research collects and uses data from previously published studies. Therefore there is no need for ethical approval. The submitted protocol was registered on the International prospective register of systematic reviews-PROSPERO CRD42023453117 (www.crd.york.ac.uk/prospero).
Search Strategy and Selection Criteria
To formulate a search question, we used the Population, Intervention, Comparator, and Outcome (PICO) framework as follows: Patients with uterine scar defects who experience symptoms and/or infertility (P), treated surgically for isthmocele (I), compared to those who remain untreated or treated non-surgically (C), with the goal of achieving symptom relief and/or conception (O). The final question is whether surgery is an effective solution for symptomatology and reproductive issues in patients with uterine scar defects and also to see which surgery technique is best for the patients.
Medical Literature Analysis and Retrieval System Online (MEDLINE) via PubMed, EMBASE (Excerpta Medical Database), Science Direct and the Cochrane Library were searched from January 2002 until June 2023, using the following medical subject heading (MeSH) and in combination with the following keywords: “cesarean scar”, “cesarean scar defect”, “niche”, “isthmocele”, “treatment”, “surgery”, “outcome”. Citation tracking was performed to identify additional publications. Our study searching protocol are presented in Supplementary Table S1.
During the study, all articles were reviewed based on their title and abstract. The inclusion criteria consisted of original articles that were randomized controlled trials, prospective observational studies with or without a control group, and retrospective cohort studies that focused on the surgical treatment of CSDs. These articles also had to clearly define isthmocele and explain how it could be diagnosed, as well as provide details on how the surgery would be performed. Conversely, article reviews, proceedings, personal comments, studies with no data on outcome interest and that only featured case reports or case series were excluded. Two investigators independently identified studies that met the inclusion criteria, and the third investigator was consulted on whether any disagreements or to resolve any differences. A discussion was conducted to make the final decision.
Data Extraction and Quality Assessment
Data extraction and quality assessment were carried out independently by two investigators. Standard forms were used to extract the following information from each study: the study authors; study design and methodology; total and mean age of the patients; type of surgery; number of previous cesarean deliveries; duration of postmenstrual spotting before treatment; isthmocele diagnosis; how the surgery performed; post operative treatment; surgical outcome. Furthermore we extracted blood loss during operation, mean operative time, intra and post operative complications, length of stay post operative, duration of menstruation post treatment in surgical outcome section. In cases of missing data in the main results or something unclear, the authors of the original publication were contacted via email.
The risk of bias assessment for included studies was conducted based on the study type. The randomized control trial study (RCT) was assessed using the Cochrane Risk of Bias tool (RoB) (9). The RoB consists of seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Other sources of bias included potential bias related to the specific study design, stopped early due to some data-dependent process, and extreme baseline imbalance. The information extracted from the paper was judged on the possible risk of bias in each domain and was rated as “low risk,” “unclear,” or “high risk.” For non-randomized studies, the risk of bias was analyzed by the Modified Newcastle-Ottawa Scale for Cohort Studies (10). The scale contains eight items within three domains. The possible total point for domain selection is 4 points, 2 points for comparability, and 3 points for outcome domain. The quality of the study was classified as “good” if the total was 7-9, “moderate” if the total score was 4-6, and otherwise as “poor.” Two reviewers independently conducted the risk bias assessment, and any disagreement was resolved by discussion with the third reviewer. The overall quality of the non-comparative studies was good with average point of 7.8 and presented in Table 1 as the risk of bias individually. Risk of bias assessment of comparative studies were available in supplement Table 2 and supplement Figure 2 for controlled trials studies.
Outcome Measurement
This study aimed to evaluate the results of surgery in isthmocele patients who experienced specific complaints, such as hip pain and abnormal uterine bleeding. The study compared the outcomes of those who received surgical treatment to those who received no treatment or treatment without surgery. Based on The
European Niche Taskforce by Jordan et al. (2019) an isthmocele, a cesarean scar defect, or uterine niche is an indentation of the uterine myometrium at the site of the Cesarean section (CS) scar with a depth of at least 2 mm and is preferably assessed by transvaginal ultrasonography (TVS) using gel or saline (11). Postmenstrual spotting was defined as two or more days of intermenstrual spotting or two or more days of brownish discharge at the end of menstrual bleeding when the total period of menstrual bleeding exceeds seven days.
Data Synthesis and Analysis Quality Assessment
The data in this study was analyzed using the MedCalc application version 22.0.01 (Ostend, Belgium) and Review Manager 5.4. The MedCalc were use for non comparable studies, the analysis was conducted using the proportion method and a forest plot was created for meta-analysis. Patient subgroups were studied separately, including those undergoing hysteroscopic, vaginal, and laparoscopic/robotic treatment for isthmocele. The proportion of patients was analyzed with a 95% confidence interval (CI). Heterogeneity was assessed using I2 statistics. A low degree of heterogeneity was considered when I2 was less than 30%, moderate between 30-50%, and high if I2 was greater than 50%. The random effects model (DerSimonian and Laird method) was used for data analysis. Additionally, the influence of individual studies on the overall results was examined.
The meta-analysis was performed using Review Manager 5.4 were performed only for comparable studies. The risks in terms of the outcomes of interest were computed and will be processed using Review Manager 5.4 and will later be presented with odds ratios (ORs) with 95% confidence intervals (CIs). Heterogeneity analysis between study populations was calculated using the I2 statistic. The I2 statistic was defined as follows, 0-24% as no heterogeneity, 25%-49% as moderate heterogeneity, 50-74% as considerable heterogeneity, and 75%-100% as extreme heterogeneity. Data are summarized across groups using the Mantel-Haenszel (M-H) for odd ratio (OR) and inverse variance for mean difference, fixed effect model if I2 < 25%. The random effect model is used if I2 > 25% (12). Funnel plots were used to evaluate publication bias. Analysis was carried out using Review Manager 5.4.
Results
Literature Search
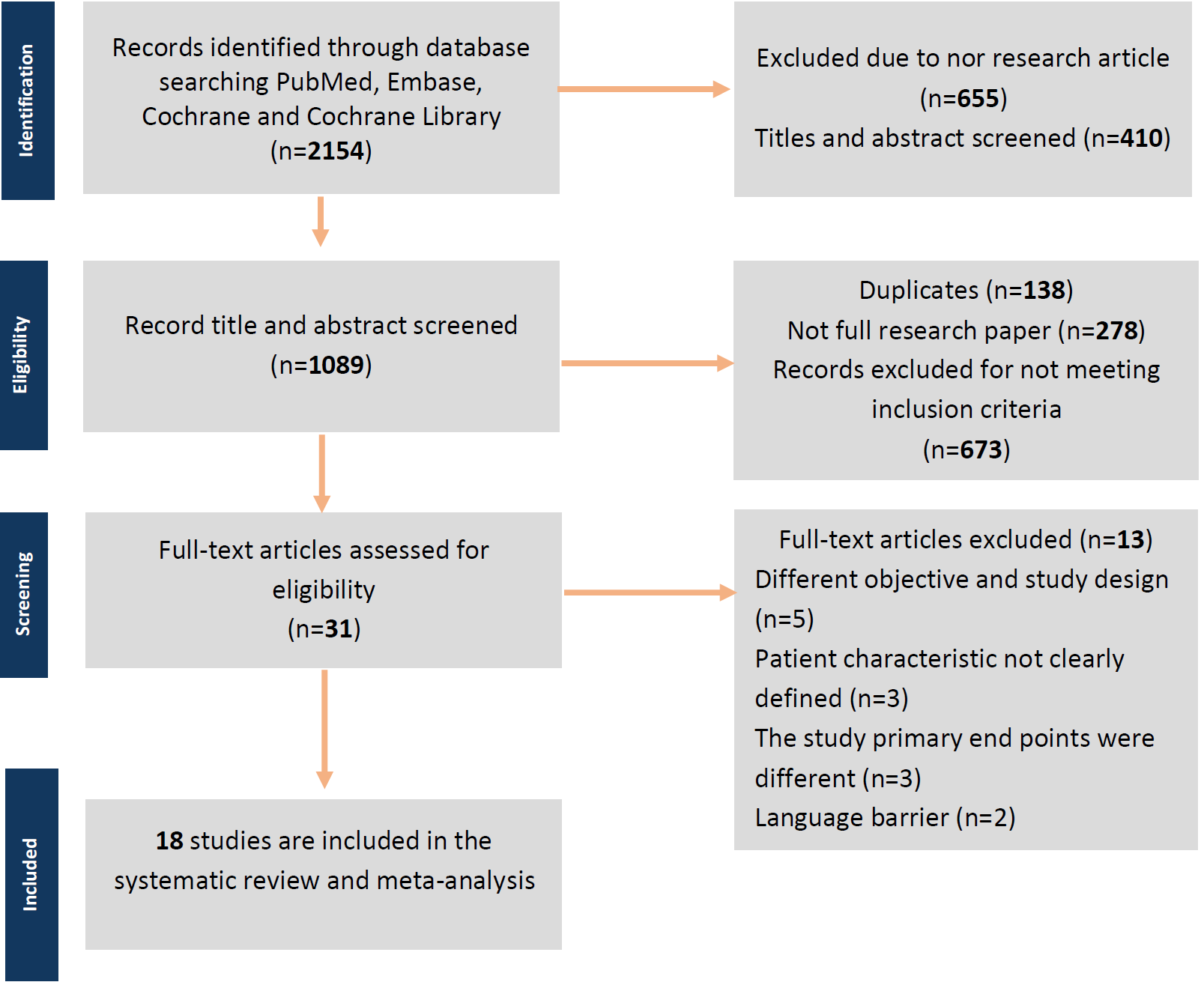
During the systematic research process, the initial screening included 2154 studies with keywords related to the research. After screening for titles and abstracts, 410 studies were excluded. An additional 655 studies were excluded as they were not original research. Of the remaining 1089 studies, 1058 were excluded for various reasons such as being duplicates, incomplete research papers, or not meeting the inclusion criteria. Thirty-one full research papers were screened, and ultimately, but only 18 studies met the inclusion criteria. The study flow diagram of the selection process can be seen in Figure 1. From 18 studies included, 1389 patients with an average age of 33.98 years old were included in the meta-analysis. Twelve non-comparative research with a total of 886 patients included had an average age of 33.25 years old (13-23), while six comparative research with a total of 503 patients that included had an average age of 34.72 years old (24-29).
Characteristics of Included Studies
This meta-analysis is divided into two study groups: comparative and non-comparative. We collected data for the table of base characteristics, including study type, surgery type, number of patients included, mean age with standard deviation (SD), number of previous cesarean deliveries, duration of postmenstrual spotting before treatment, and its SD. The table for the base characteristics of non-comparative studies is presented in Table 3, while the table for comparative studies is shown in Table 4. The group was divided into two groups which were Non-comparative and comparative studies, this was carried out in order to keep the results of the meta-analysis fair and reduce selection biases. In addition, this was done because it was not possible to carry out comparisons of intervention and control when taking population samples from different studies. Non-comparative studies were subject to proportion analysis as they only focused on one intervention. On the other hand, a forest plot of pooled comparative analysis was conducted for comparative studies, which had both intervention and control results within a specific population. In this study we also make a table summary for how the isthmocele were diagnosed in each study, how the surgery was performed, and were there any post operative treatment were given, the table were available in supplement table 5 for non-comparative studies and table 6 for comparative studies.
Out of the total twelve studies in the non-comparative study, eight studies (66.7%) were conducted in Asia, 3 studies (25.0%) in Europe, and one study (8.3%) in South America. Laparoscopic surgery was the focus of three studies, which included a total of 120 patients. Hysteroscopic surgery was the focus of four studies, with 169 patients included. Five studies were focused on transvaginal surgery, which included as many as 597 patients. Of the six comparative studies, four studies (66.7%) were from Asia and 2 studies (33.3%) were from Europe.
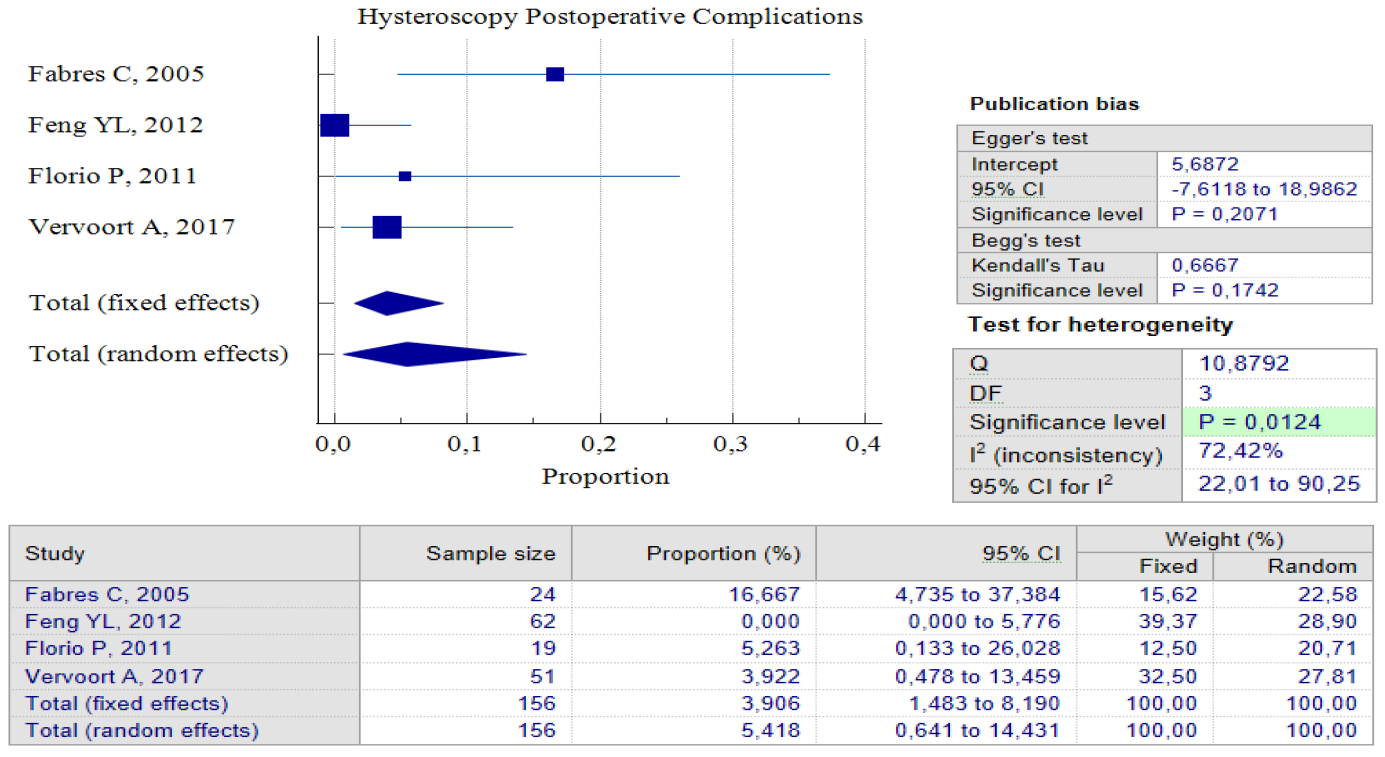
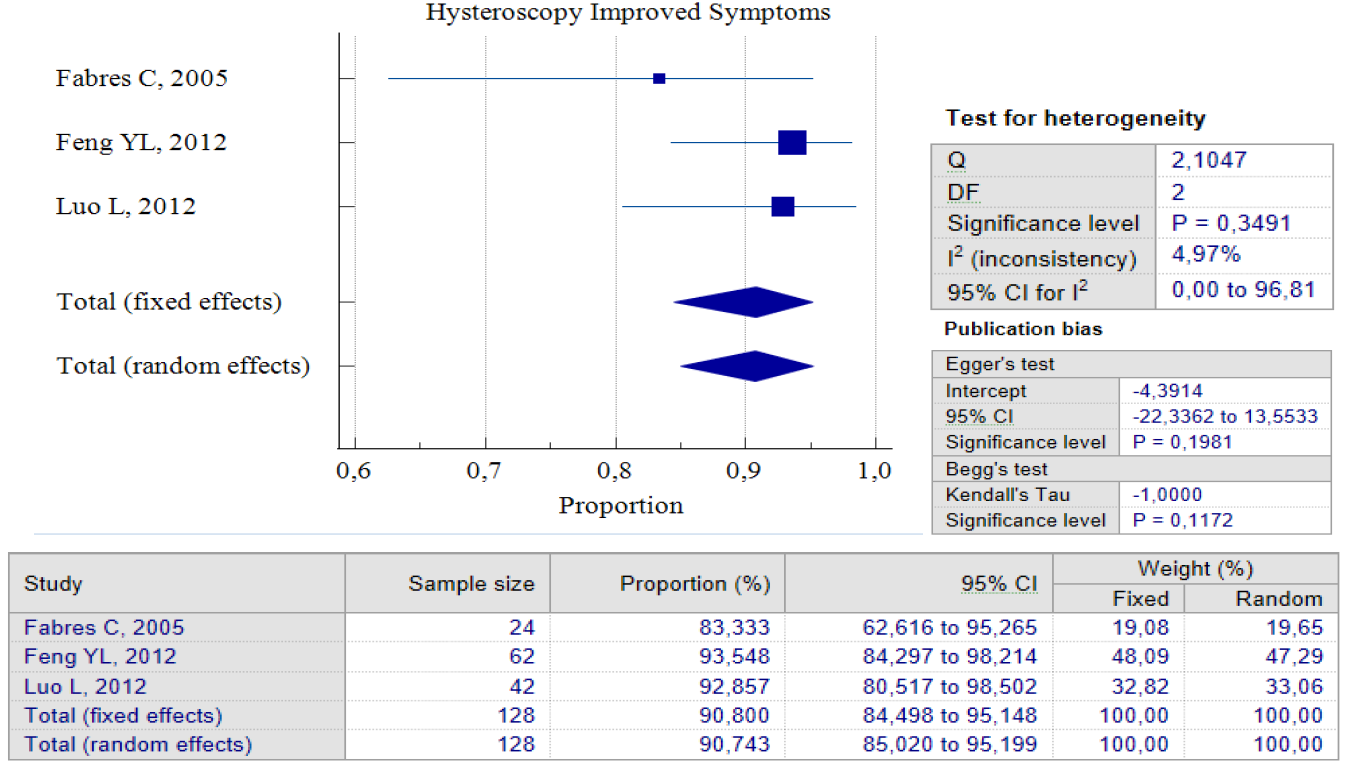
Data Synthesis: Non-comparative studies have consistently shown that surgery yields better outcomes when compared to no treatment. The result of pooled proportion analysis of these studies revealed that hysteroscopy, particularly, had better outcomes in addressing symptoms, with up to 90.80% of patients reporting an improvement after undergoing the procedure, 95% CI (84.49 – 95.15) and low heterogeneity (I2 = 4.97%), which indicates that all studies produced similar results. In this case, symptoms referred to abnormal postmenstrual uterine bleeding and pelvic pain. Hysteroscopy treatment improved these symptoms with low postoperative complications, with only 5.4%, 95% CI (0.64 – 14.43) I2 = 72.42%. In this case, postoperative complications refer to complications such as Ashermann syndrome, ectopic pregnancy, or vesico-uterine fistula. Figures 3 and 4 provide pooled proportion analyses of postoperative complications and symptom improvement of symptomatic isthmocele patients after hysteroscopy surgery.
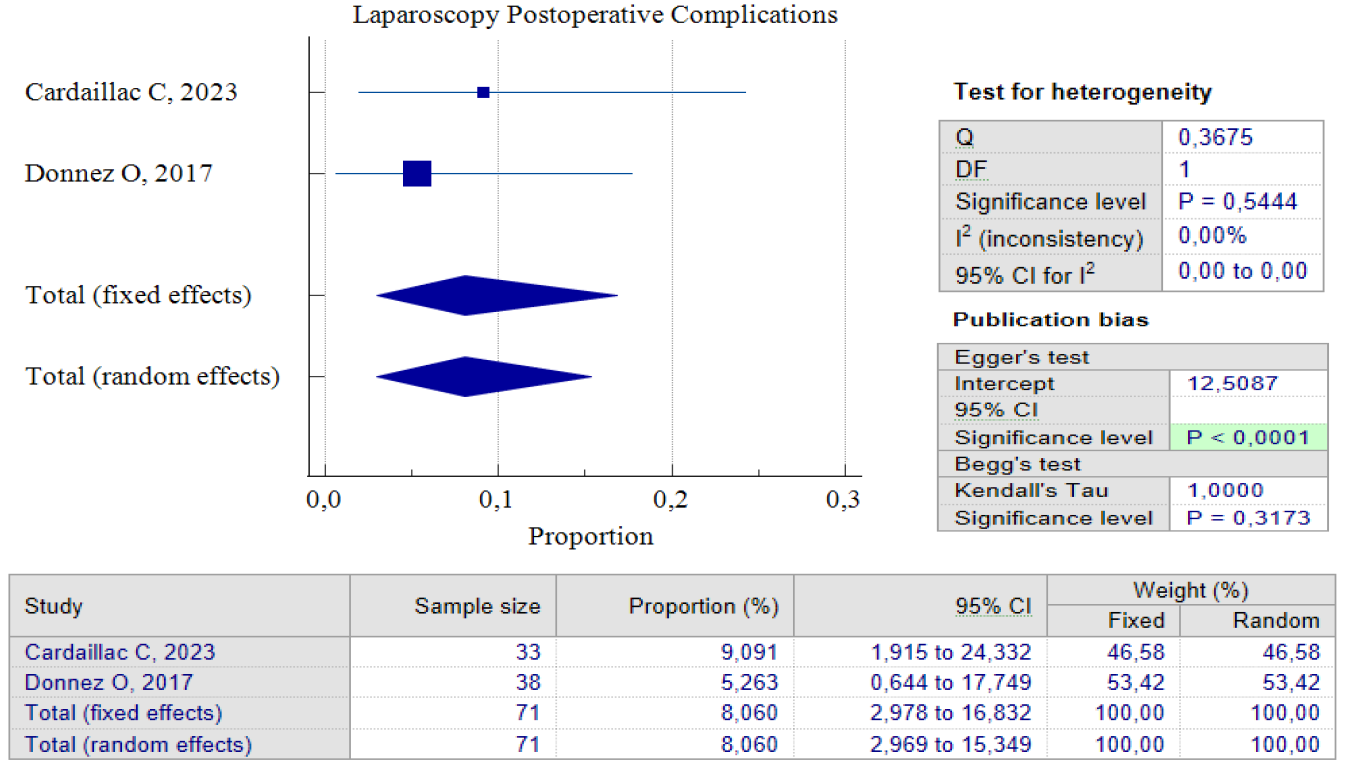
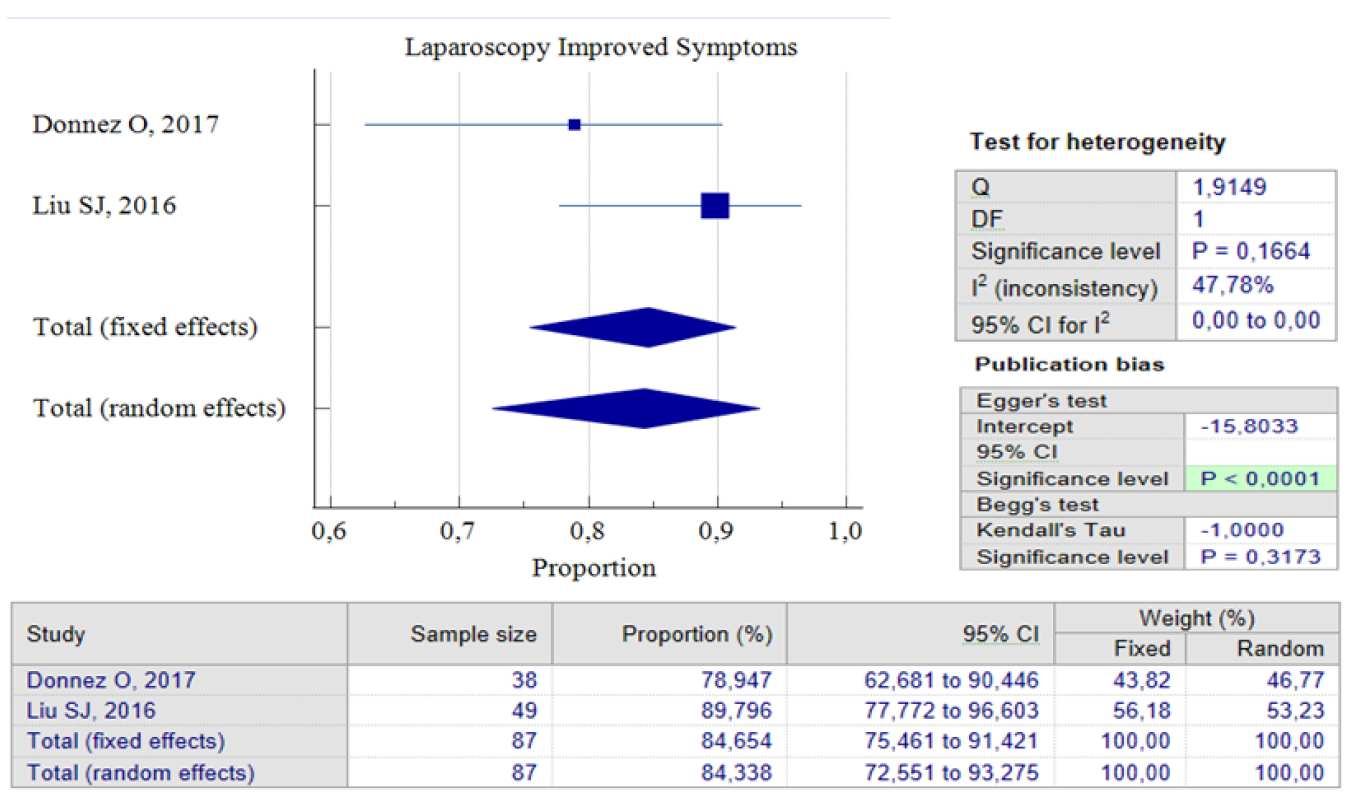
When it comes to laparoscopic procedures, there are only three studies available for proportional analysis. Unfortunately, only two studies can be analyzed when it comes to the analysis of improved postoperative symptoms. This study utilized the random effect model method to account for the small number of studies and moderate heterogeneity found between the studies. This method helps to eliminate bias factors from the sample size in each study. Our analysis found that 84.34%, 95% CI (72.55 – 93.27) and an I2 of 47.78%, have improved symptoms postoperative. These improvements were related to the duration of postmenstrual abnormal uterine bleeding and pelvic pain. Interestingly, both studies show that postoperative complications related to laparoscopic procedures are relatively small. Only 8.06% of patients reported complications after the procedure, with 95% CI (2.97 – 16.8) I2= 0.00%. It’s worth noting that the analysis results show low heterogeneity, but this could be due to the limited number of studies included. Figures 5 and 6 provide pooled proportion analyses of postoperative complications and symptom improvement of symptomatic isthmocele patients after laparoscopy surgery.
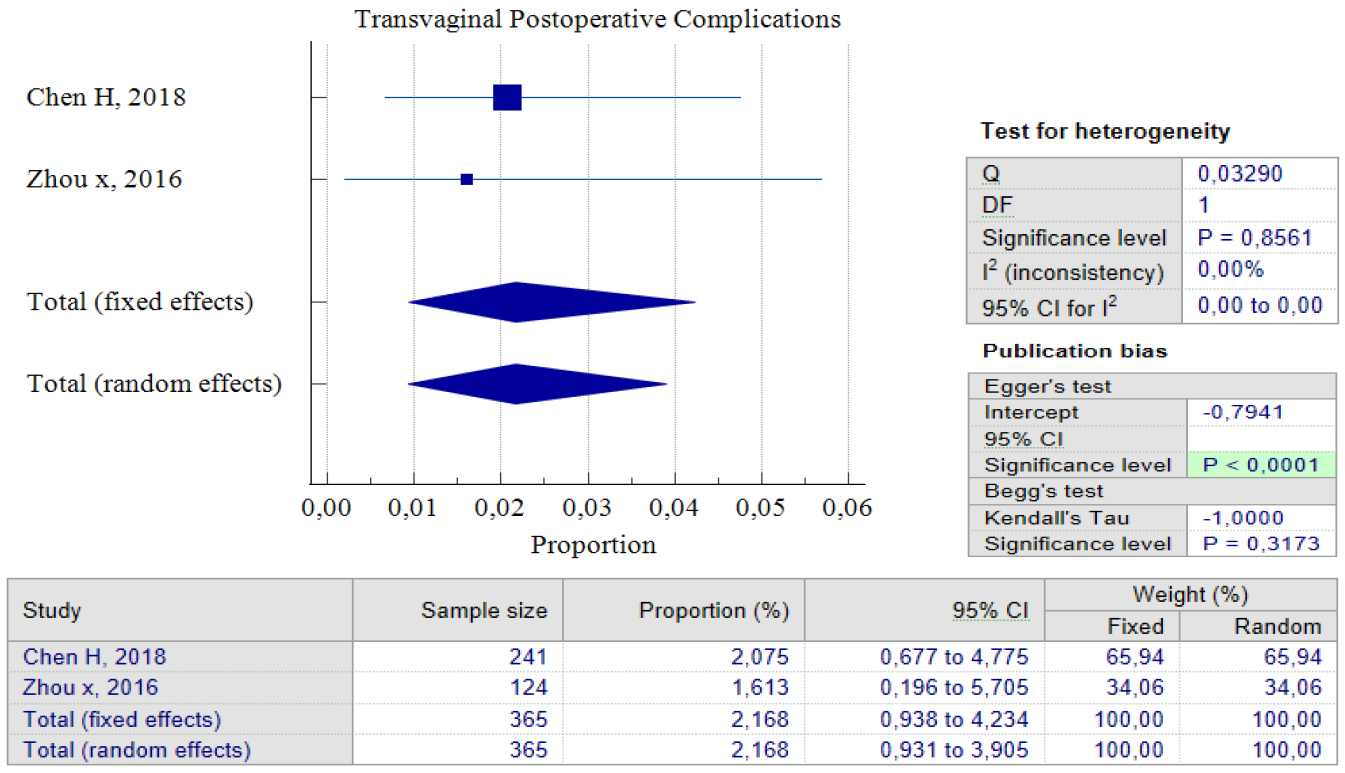
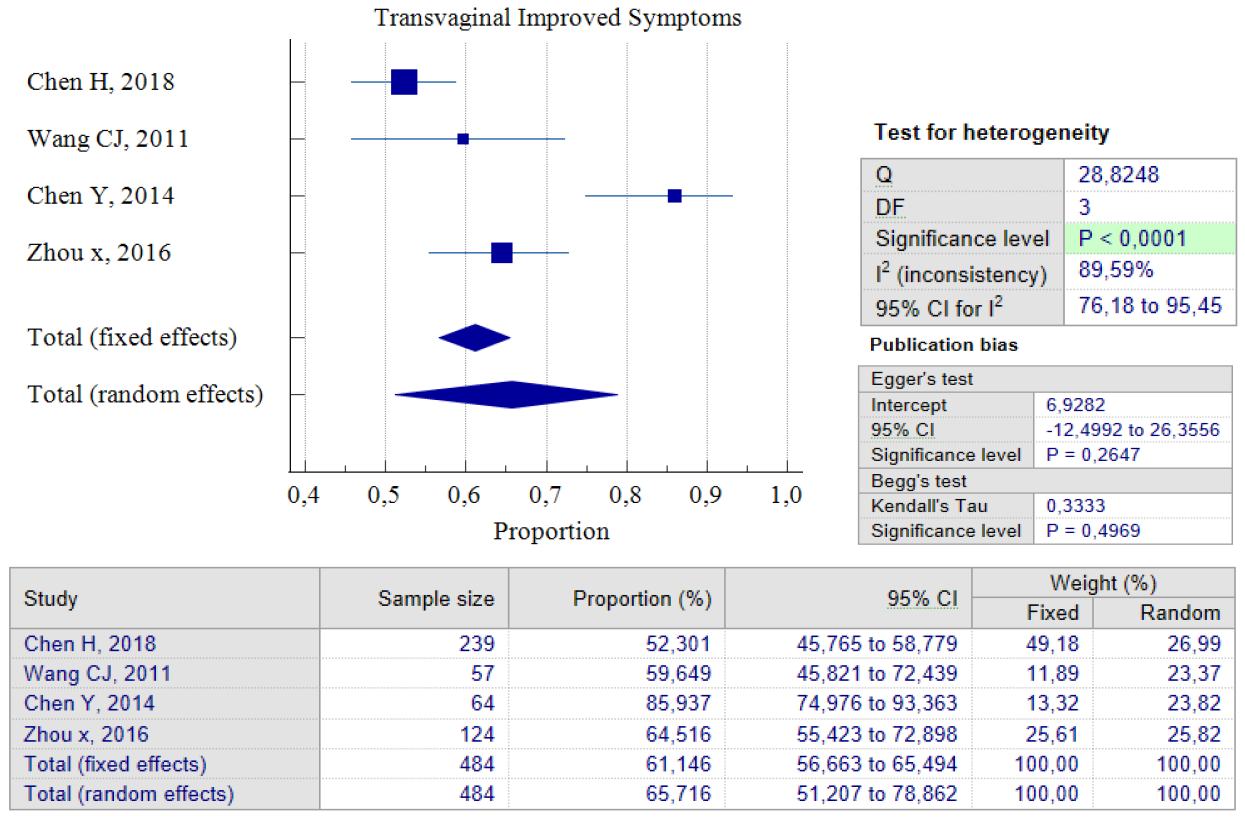
Out of the five studies that concentrated on transvaginal surgery, only four of them could carry out pooled proportion analysis. The reason for this was that one study by Zhou J, 2016 used different units of variables from the other researchers. As a result, we chose not to include that particular study in the analysis. According to the findings of the pooled proportion analysis, compared to the two earlier surgical techniques, patients who underwent isthmocele treatment using the transvaginal surgery technique had the lowest proportion of improved symptoms, which was 65.71%, with a 95% CI of (51.20 – 78.86) and I2 = 89.59%. However, it is worth noting that this technique has a low rate of postoperative complications, which was 2.17%, with a 95% CI of (0.93 – 4.23) and I2 = 0.00%. The low complication rate could be as a result of the fact that only two studies were used for pooled analysis. Additionally, transvaginal surgery is a classic technique and the oldest surgical method, which most practitioners are familiar with. Figures 7 and 8 provide pooled proportion analyses of postoperative complications and symptom improvement of symptomatic isthmocele patients after transvaginal surgery.
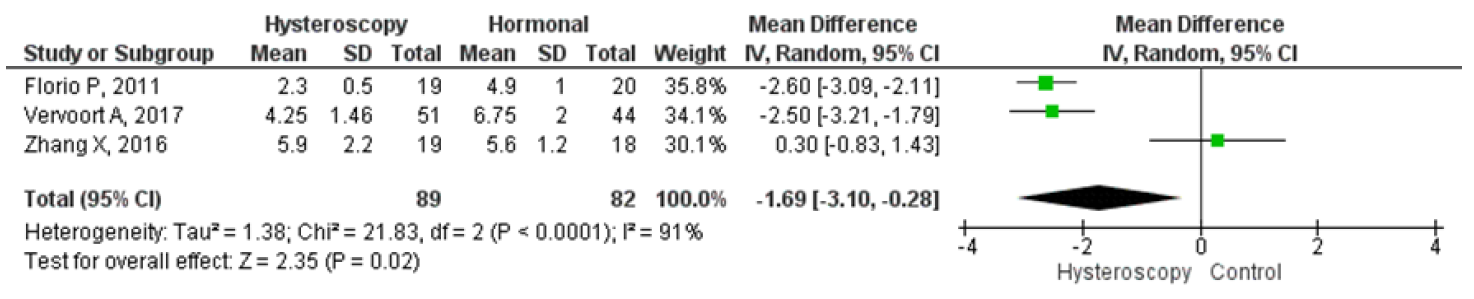
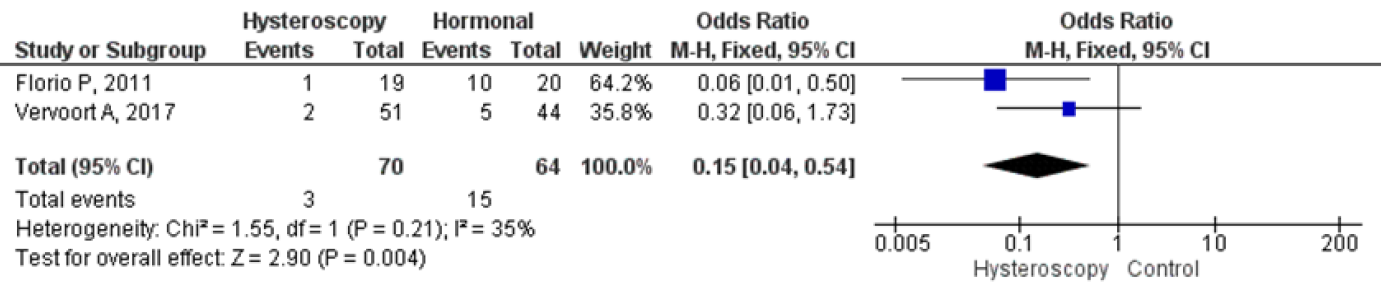
Four studies were analyzed for comparing hysteroscopy and hormonal treatment. The pooled analysis showed that hysteroscopy had significantly better outcomes in reducing the mean duration of menstruation which were in days. The forest plot indicated a lower mean difference (MD) of 1.69, 95% CI (0.28 – 3.10), and a p-value of 0.02, while I2 was 91%. Two studies compared postoperative pelvic pain between hysteroscopy and hormonal treatment, and the forest plot showed that the proportion of patients experiencing postoperative pelvic pain was significantly lower in hysteroscopy with an odds ratio (OR) of 0.15, 95% CI (0.04 – 0.54), and a p-value of 0.004, while I2 was 35%. Figures 9 and 10 display a forest plot comparing the mean duration of menstruation and pelvic pain post-treatment between hysteroscopy surgery and hormonal therapy.
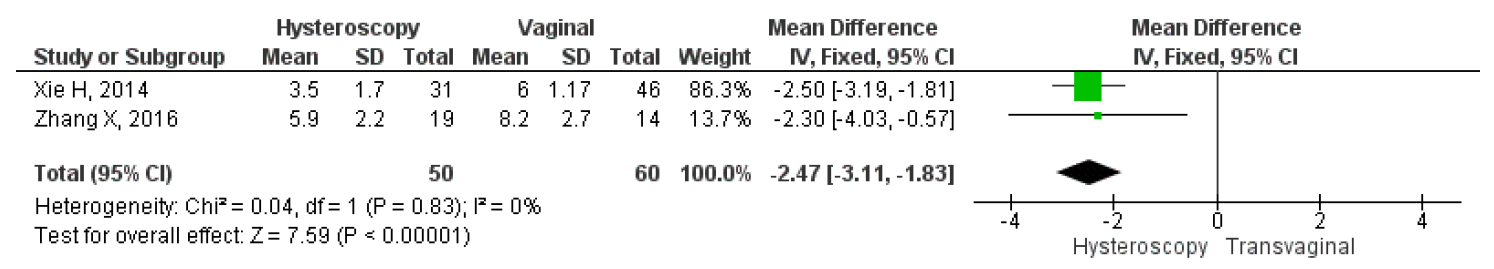
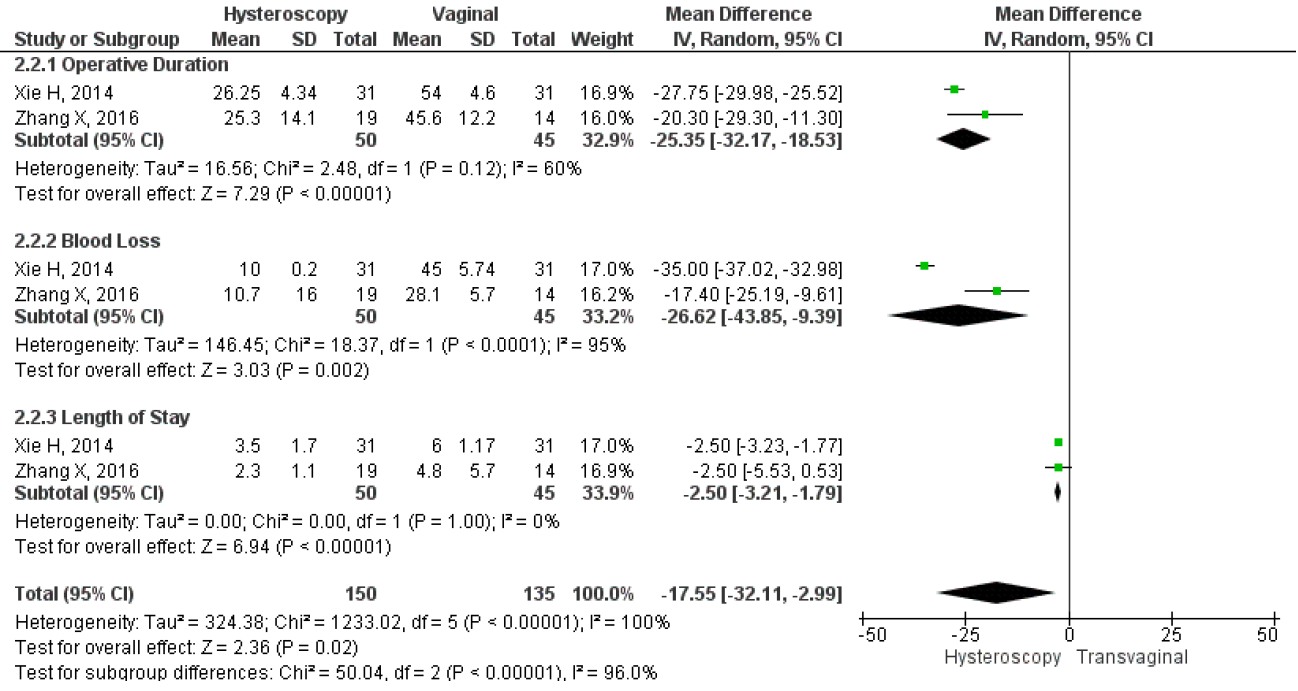
However, only two studies directly compared the mean duration of menstruation after treatment, intraoperative, and postoperative complications between hysteroscopy and vaginal surgery. The results showed that hysteroscopy had a significantly lower mean duration of postoperative menstruation, MD 2.37, 95% CI (1.83 – 3.11), and a p-value of less than 0.001. Heterogeneity was low, with an I2 of 0%, fixed method was used due to the low heterogeneity found in the studies. Figure 11 shows a forest plot comparing the mean duration of menstruation post-treatment between hysteroscopy and vaginal surgery. Furthermore, this study analyzed intraoperative and postoperative outcomes between hysteroscopy and transvaginal surgery. For intraoperative outcomes, we analyzed the duration of the operation and the amount of blood loss during surgery, which were in min and ml respectively. For postoperative, we analyzed the length of stay post-surgery which were in days. The study also compared the results of each intraoperative and postoperative outcome between the surgical techniques to see which one had the best overall surgery outcomes. Our analysis showed that hysteroscopy had significantly lower operative duration MD 25.35, 95% CI (18.53-32.17), p<0.001, I2=60%; lower mean blood loss MD 26.02, 95% CI (9.39-43.85), p=0.002, I2=95%; and also shorter length of stay MD 2.5, 95% CI (1.79-3.21), p<0.001, I2=0%. Overall, the forest plot showed that hysteroscopy had better outcomes for both intraoperative and postoperative complications. The forest plot may be seen in Figure 12.
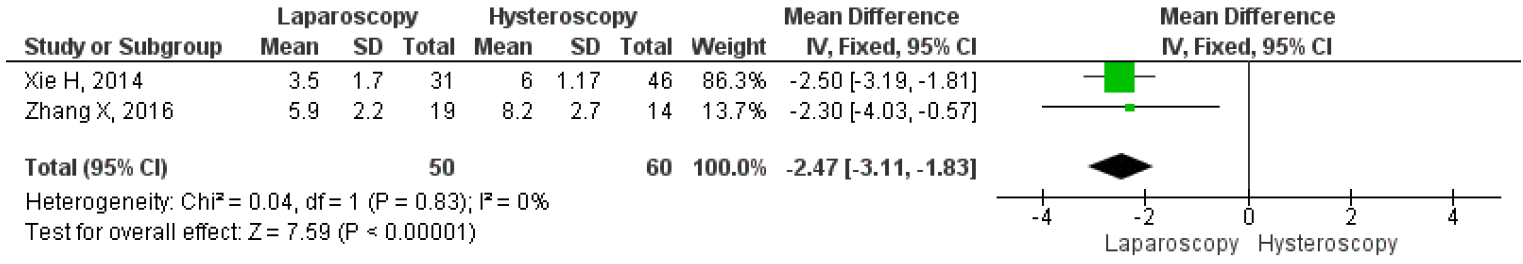
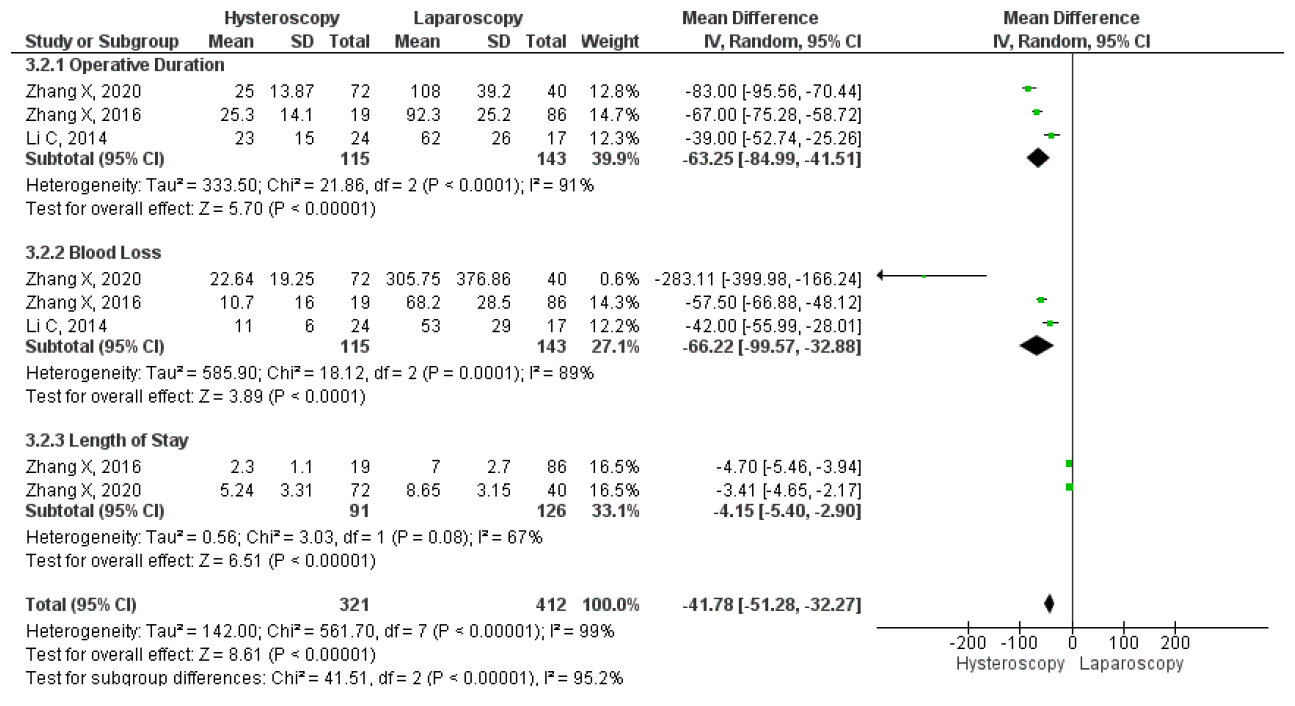
Unfortunately, only two studies compared the mean duration of menstruation after laparoscopy and hysteroscopy surgeries. The results on the forest plot indicated that the laparoscopic procedure led to a significantly shorter mean duration of menstruation post-treatment, with an MD 2.46, 95% CI (1.83 – 3.11) p <0.001, I2=0%. Figure 13 displayed the forest plot, which compared the mean duration of menstruation post-treatment between laparoscopy and hysteroscopy surgeries. Furthermore, three studies were included that compared the intraoperative and postoperative outcomes of each technique. Hysteroscopy was found to have significantly better outcomes for operative duration with an MD 63.25, 95% CI (41.51 – 84.99) P<0.001, I2=91%; lower mean blood loss, with an MD 66.22, 95% CI (32.88 – 99.57) p<0.001, I2=88%; and shorter length of stay, with MD 4.15, 95% CI (2.90 – 5.40) p<0.001, I2=67%. The forest plot in Figure 14 showed that although laparoscopy had a better outcome in terms of mean duration of menstruation post-treatment, the procedure had inferior intraoperative and postoperative measures if seen in operative duration, blood loss, and length of stay compared to hysteroscopy.
Discussion
Isthmocele is a myometrial defect or a hypoechoic triangle in the anterior uterine wall at the site of hysterotomy presented in transvaginal ultrasound (TVUS) or sonohysterography (SHG) examination in non-pregnant women following a previous cesarean scar CS (30,31). The location of the defect varies depending on the location of the CS, the stage of labor, changes in the uterine cervix, and the surgical technique (32). The isthmocele indicates insufficient healing of the myometrium at the location of a cesarean incision. The phenomenon’s prevalence exhibits significant variation, ranging from 6.9 to 69%, contingent upon the characteristics of the studied population and the employed methodology (33). According to the findings of Antila-Långsjö et al., the prevalence of isthmocele following elective caesarean delivery (CD) was 41.4%, while it was found to be 58.6% in cases of emergency CD. This finding demonstrates a higher incidence of isthmocele in emergency cesarean deliveries than in elective cesarean deliveries. The primary causes for Emergency Cesarean Deliveries were prolonged labour, 44.0% of cases, and fetal hypoxia, accounting for 32.4%. A total of 371 women were included in the study, among whom 59 cases (15.9%) were diagnosed with intrapartum or postoperative infection. Their prospective observational cohort study included patients who were recruited either prior to undergoing the cesarean delivery procedure or within three days following the procedure.(34) Sonohysterography (SHG) has been found to possess a higher level of accuracy in the diagnosis of niches. The use of sonohysterography (SHG) has been found to result in a higher proportion of identified abnormalities (45% opposed to 22%) when compared to transvaginal ultrasound (TVS). Additionally, SHG has demonstrated the ability to detect larger niches and thinner residual myometrium. Additional specialized characteristics, such as concavity, atypical vascularity, visible serosa, and the presence of cysts or polyp-like structures, should also be included in the discussion. Superficial high-resolution ultrasound imaging (SHG) conducted at 6-12 weeks after childbirth, when the surgical incision has not fully healed, assists in the identification of scar tissue and minor indentations. This process is further supported by the presence of a thin endometrium during breastfeeding (35).
The study found that there was no notable difference in the incidence of isthmocele between emergency and elective CD OR 1.03, 95% CI (0.68 – 1.56). However, it did reveal a significant relationship between previous CD and isthmocele development OR 3.69, 95% CI (2.38 – 6.04) p<0.001. Furthermore, the multivariate analysis for emergency CD indicated that longer active labor before emergency CD was associated with a higher risk of isthmocele development OR 1.06, 95% CI (1.01 – 1.11) p=0.032. This factor was found to be an independent risk factor for isthmocele development. Hayakawa H et al. also discovered a similar outcome. A study conducted in Japan in 2006 revealed that there was no notable difference between the risk of elective or urgent CD and the incidence of isthmocele. The study also examined the correlation between isthmocele and suturing techniques. Follow-up was conducted one month after the procedure.
The study found that the lowest incidence of isthmocele was observed when using the single-layer myometrium closure with decidual suture technique OR 0.077 (0.012 – 0.49) p=0.007, followed by double-layer myometrium close OR 0.28 (0.085 – 0.94). However, no significant differences were found for single-layer myometrium closure. The study suggests that the disadvantage of a continuous single suture not precisely joining the tissues together must be weighed against the similar short-term maternal morbidity with a shorter operative period reported for single-layer closure, which explains its broad clinical acceptance (36).
Antila-Långsjö study also identified infections encompassed chorioamnionitis, endometritis, and postpartum wound infections. The diagnostic criteria for chorioamnionitis encompassed intrapartum fever and increased infection markers (such as C-reactive protein and leukocyte count), accompanied by maternal or fetal tachycardia (33). Various researchers have employed different timeframes for conducting clinical examinations to diagnose isthmocele. According to the findings of Gozzi et al., the prevalence of isthmocele, as determined by transvaginal ultrasound (TVS), was shown to be 44.4% among women who had previously undergone cesarean section (CS) six months prior (37). Dosedla et al. selected the time points of 6 weeks and 18 months postpartum to investigate the clinical manifestations of isthmocele (38). In contrast, previous studies explored isthmocele within varying time intervals of 6-12 weeks, 6-9 months, or 6-12 months postpartum, resulting in a more diverse timeframe and differing definitions of a niche (39). According to research conducted by Hayakawa H et al, there is a higher risk of isthmocele associated with premature rupture of membrane (PROM) OR 8.72, 95% CI (1.28-59.65) p=0.027 (35). Similarly, Park et al found a correlation between the incidence of isthmocele and PROM in a 2018 case-control study from Korea with OR 1.90, 95% CI (1.08-3.34) p=0.027. It is important to note that PROM is a significant risk factor for infection and can weaken the healing process of uterine closure. Patients with PROM may have an immature lower segment of the uterus, which can negatively impact the suture and wound healing process. Appropriate management should be implemented if PROM occurs during delivery to prevent infection and ensure proper healing and remodeling of the myometrial incision. Interestingly, one study has indicated no notable difference in the occurrence of isthmocele between urgent or elective cesarean deliveries in relation to infections like chorioamnionitis, postpartum wound infections, and endometritis (34,36). The disparities in the findings of this study could be attributed to the varying approaches to infection management across different centers, as the treatment of infections may impact the process of remodeling of the myometrial incision. It is hoped that appropriate treatment of postoperative endometritis or myometritis would foster optimal healing.
Isthmocele has the potential to be classified as one of the primary etiological factors contributing to infertility. An association was observed between the inability to conceive and an increased prevalence of cesarean deliveries. Calzolari et al. conducted a study in which patients were categorized into two groups: fertile patients, who achieved pregnancy within 12 months after diagnosis of isthmocele, and infertile patients, who did not achieve pregnancy within the same timeframe. The authors reported that the association between BMI and infertility supports previous findings in the literature (40). Isthmocele may contribute to developing a uterine cavity that is physically less conducive to fertility. Additionally, it was revealed that the individuals who could not achieve pregnancy within a 12-month following the surgical procedure exhibited advanced age and a more severe isthmocele grade compared to those who could conceive. Age is a widely recognized risk factor for infertility, and earlier research has demonstrated a correlation between the severity of isthmocele, cervical dilatation, and infertility (40,41).
Currently, there are no diagnostic criteria that have been universally agreed upon (41). Some authors classified isthmocele based on the size of the defect, with a large defect if the myometrial reduction of >50% of the wall thickness or even >80% (42). In addition, residual myometrium(RM) 2.2 mm by TVUS and 2.5 mm by SHG may also describe a large defect (43). Marotta et al. adopted the cutoff of RM >3 mm as a large defect, and RM <3 mm as a small defect for management purposes (44). In the absence of symptoms, these radiologic findings may be discovered by chance or linked to clinical signs. They can therefore be categorized as either asymptomatic or symptomatic when presenting with abnormal uterine bleeding (AUB), pelvic pain, or infertility (45,46).
The treatment options for isthmocele include expectant or pharmacological treatment and surgical treatment such as hysteroscopic, laparoscopic, laparotomic, or transvaginal procedures (47). The treatment decision is based on the size of the defect, symptoms, secondary infertility, and pregnancy plans (29,48). Clinical observation and no surgical intervention are usually recommended for an incidental diagnosis of asymptomatic isthmocele and no plans for future childbearing (44). Numerous studies propose various surgical approaches for correcting the cesarean scar defect. Several studies have suggested that surgical management can help reduce symptoms and improve obstetric outcomes (49). The surgical procedure generally involves resectioning fibrotic tissue from the defect, presented as a flap beneath the triangular pouch. Resection of the isthmocele edges, which connects the wall to the cervical canal, improves flow drainage and prevents menstrual blood retention (46). More advanced cases of isthmocele with RMT < 50% or <3mm usually require surgical resection through vaginal, laparoscopic, laparotomic or combined (abdominal/transvaginal) approaches (50). The vaginal approach to isthmocele repair is technically difficult due to the limited field of the operation. This technique requires the bladder preparation, opening the vesicovaginal space and resection of fibrotic cesarean scar. Intraoperative identification of the defect is the most difficult part of the operation. Additional devices like hysteroscope (backlight), Foley catheter, or Hegar dilators (“slip and hook” technique) are very useful for the mapping the defect during these procedures. The gentle tissue preparation and non-coagulating cutting, followed by two-layer closures (2-0 thread) appeared to be the most effective way of treatment. The improvement of reducing the symptoms was reported in nearly 90% (51,52).
Nonetheless, data on which surgical approach best suits patients with symptomatic isthmocele still needs to be clarified. Further in this systematic review and meta-analysis, we evaluate the benefits and challenges of hysteroscopy, laparoscopy, and transvaginal surgery approaches. The hysteroscopic surgery approach is a minimally invasive, non-time-consuming, and low-morbidity procedure that allows direct visualization and repair of the defect (30). The hysteroscopic management targets the simple triangular pouch with a minimum residual myometrium of 2.5-3 mm (48). It entails coagulating its roof following resection of either the distal edge alone (Fabre’s technique), the distal and proximal edge (Gubbini’s technique), or the whole canal by channel-like 360° resection (45). The pooled proportion analysis in our study showed an improvement of symptoms in 90.74% (75.02% – 95.19%) after hysteroscopic surgery. Our results also showed that hysteroscopy had better outcomes in operation duration, blood loss, and length of hospital stay than another surgery approach. A systematic review by Abacjew-Chmylko et al. reported 85.5% favorable outcome rates for hysteroscopic resection, ranging from 59.6% to 100%, with 72.4% of cases completely resolving AUB symptoms.46 The main risks of the hysteroscopic procedure are uterine perforation and bladder injuries. Resectoscope treatment by hysteroscopy is recommended to reduce this risk if the remaining myometrial thickness is greater than 3 mm (50).
Laparoscopic isthmocele repair is performed with excision of the scar tissue from the edges of the isthmocele followed by closure of the defect with sutures in two layers, the first being interrupted monofilament sutures. Laparoscopy provides optimal visualization in identifying the isthmocele and allows for the repair and consequent increase in myometrial thickness (53-55). Donnez et al. examined gynecological, obstetrical, and myometrial outcomes after laparoscopic isthmoceles repair (15). MRI-detected symptomatic isthmoceles with myometrial thickness less than 3mm were treated laparoscopically in 38 individuals. The Lumenis-Sharplan CO2 laser was used for laparoscopic isthmocele removal. After excising the isthmocele, a Hegar probe was placed into the cervix to maintain cervical canal-uterine cavity integrity. A three-layer incision repair followed. After closing the first two layers with Vicryl sutures, the surgeon closed the peritoneum with Monocryl running sutures. Vervoort et al. advised shortening round ligaments when the uterus was retroflexed to decrease counteracting forces that could hinder wound healing. Hysteroscopy checked the repair, and histology examined the isthmocele (25).
After undergoing surgery, an MRI was done three months later to assess the thickness of my myometrium. The results showed that the mean thickness of my myometrium had increased from 1.43 0.7 to 9.62 1.8 mm. Additionally, 91% of patients reported being symptom-free, and among women who had infertility issues, 44% were able to conceive and deliver healthy babies at full term successfully. The significant increase in myometrial thickness demonstrates the effectiveness of undergoing a laparoscopic isthmocele repair in restoring the anterior uterine wall (15). If a patient has enough residual myometrial thickness over their isthmocele (RM >3 mm), hysteroscopic correction might be thefastest, easiest, and safest surgical treatment. However, if a patient has thinner residual myometrial thickness (RM < 3 mm), laparoscopic correction is a better option to avoid the risk of uterine perforation and bladder injury. A study from Belgium with the mean value of the residual myometrial thickness in the study population of 1.77 mm reported that the laparoscopic approach was the preferred method (15,56-58).
In addition, using a laparoscopic surgical approach can increase the amount of myometrial tissue left for patients who plan to have children in the future. Although there is a significant reduction in myometrial thickness one month after surgery, compared to three to six months after surgery, this does not indicate that the therapeutic effect of the surgery has decreased over time. The thickness of the myometrial tissue is still significantly greater than the average thickness before surgery (p-value <0.001). This may caused by the resolution of the inflammatory changes and edema of the uterine myometrium at the surgical site (59). In this meta-analysis, it was found that the laparoscopic technique had a high success rate in improving patient outcomes (84.33%, 95% CI 72.55 – 93.27), second to only hysteroscopy (90.74%, 95% CI 85.02 – 95.19). Additionally, a pooled analysis using the Inverse Variance method demonstrated that laparoscopy is also more effective in reducing the length of menstruation after surgery with MD lower 2.47, 95% CI (1.83 – 3.11) when compared to hysteroscopy. However, it is important to note that this analysis is limited by a small number of studies on laparoscopic techniques, emphasizing the need for further research to confirm these findings (60).
Minimally invasive surgery has become more popular due to its advantages over invasive techniques. It offers improved safety and a shorter recovery period. However, this procedure requires special equipment and may not be available in underdeveloped or medically underserved areas. Vaginal surgery, a traditional operative procedure, has been widely used for a long time and most gynecologists are skilled in its techniques (56). According to a retrospective study, both techniques had similar outcomes. The first technique involves dissecting the bladder from the cervix and uterus, then opening the vesicovaginal space to identify the isthmocele. The defect is removed, and the hysterotomy is closed in two layers. On the other hand, transvaginal isthmocele repair was found to be more cost-effective and has shorter operation time than laparoscopy. However, this method requires a surgeon who is highly experienced in vaginal surgery to avoid damaging adjacent structures and to accurately locate the isthmocele in the limited surgical view (61). It is crucial for obstetricians to be aware of the numerous factors that come into play when treating isthmocele, such as lost follow-ups after cesarean delivery, suturing technique, surgeon performance, and infection management. However, there are currently no established guidelines for determining the appropriate timing of follow-up and intervention. Some studies suggest a follow-up period of 3-6 months after the procedure, based on the understanding that wound healing takes at least six months. An earlier assessment may lead to overdiagnosis, but a delayed evaluation may result in a higher dropout rate due to subsequent pregnancies. Treatment is only recommended for symptomatic women with secondary infertility, previous ectopic scar, recurrent miscarriage, AUB, and bothersome post-menstrual spotting. The efficacy of treatment has yet to be confirmed. It is not recommended to routinely repair an incidentally diagnosed isthmocele in women who do not plan on having future children. According to the hypothesis proposed by Vervoort et al, there are several potential factors that may contribute to the development of isthmocele, a defect in the cesarean scar. These factors include a low incision through the cervical tissue, suboptimal suturing or incomplete closure of the uterine wall, and surgical techniques that promote adhesion formation. Specifically, non-closure of the peritoneum, insufficient hemostasis, and visible sutures may all contribute to this complication (62, 63).
In a recent study, it was discovered that utilizing a single full-thickness closure technique resulted in more complete closure than the single-layer decidua sparing closure technique. Interestingly, almost 95% of patients with isthmocele undergo single-layer closure without closing the peritoneum. The risk of isthmocele can be minimized by creating a strong myometrial scar through proper anatomical approximation without tissue strangulation. For optimal healing and lower risk of niche formation, it is best to include the deeper part of thick muscular edges in the first layer and the remaining superficial cut edges in the second layer. Non-perpendicular sutures, locking sutures, or a very tight second layer can lead to irregular myometrial closure and ischemic necrosis, resulting in poorly healed scars. The recommended technique for uterine closure is double-layer using non-locking sutures, which allows for thicker residual myometrium. Suboptimal surgical techniques including inadequate hemostasis, tissue ischemia, devascularization, and excessive tissue manipulation can contribute to poor scar healing and adhesions, ultimately forming an isthmocele (64,65). Prior to selecting a specific surgical approach, it is recommended to conduct a thorough preoperative evaluation with transvaginal ultrasound, sonohysterography, and/or magnetic resonance imaging (MRI) in order to examine the extent of myometrial coverage over the isthmocele. When considering the option of undergoing surgery, it is of utmost importance to consider the remaining myometrial thickness that encompasses the isthmocele in those who are experiencing symptomatic isthmocele.
Strength and Limitation
As far as we know, our meta-analysis is the first to assess comparative studies on how effective various surgical approaches are for treating isthmocele. However, our findings are limited due to the small number of patients in most studies, variations in patients’ characteristics, poor quality of original research, and concerns about how isthmocele was diagnosed in different studies. During our comparative studies analysis we also encountered an obstacle where each study had different variables. This hindered us from making a proper comparison, despite all studies having the same primary outcome. Additionally, surgeons’ varying skill levels may also affect the results of particular surgical techniques. These factors must be considered when weighing any surgical approach’s benefits and risks.
Conclusion
According to this meta-analysis, surgery is a viable treatment option for patients experiencing symptomatic isthmocele. Over 65% of patients report improved symptoms after undergoing surgery. For patients with adequate residual myometrial thickness overlying the isthmocele (≥ 2.5–3.5 mm), hysteroscopic correction may be the safest and most effective strategy. However, for those with a thinner residual myometrium over the defect (< 2.5 mm) or when hysteroscopic treatment is inconclusive, laparoscopic and vaginal surgeries may be the preferred options. This study showed that laparoscopic procedures have better outcomes compared to hysteroscopy. However, there are some challenges during the surgery, such as longer duration, increased blood loss, and longer hospital stay. Moreover, some centers or countries may have financial and equipment limitations. Nonetheless, vaginal surgery is still a feasible alternative in situations where resources are limited.
To summarize, it is still necessary to conduct high-quality comparative studies to determine the best surgical approach for treating symptomatic isthmocele. These studies will also help establish a specific threshold for residual myometrial thickness, below which hysteroscopic surgery should not be performed. Additionally, further research is needed to explore the potential benefits of surgical correction for asymptomatic isthmocele, including improved fertility and reduced risk of obstetrical complications.
References
Figure 1. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) flow diagram
Figure 2. Proportion of Hysteroscopy Postoperative Complications
Figure 3. Proportion of Hysteroscopy Improved Symptoms Post Treatment
Figure 4. Proportion of Laparoscopy Postoperative Complications
Figure 5. Laparoscopy Improved Symptoms Post Treatment
Figure 6. Transvaginal Surgery Postoperative Complications
Figure 7. Transvaginal Improved Symptoms Post Treatment
Figure 8. Pooled Analysis of Mean Duration of Menstruation Post Treatment Between Hysteroscopy Surgery and Hormonal Therapy in Patients with Symptomatic Isthmocele
Figure 9. Pooled Analysis of Pelvic Pain Post Treatment Between Hysteroscopy Surgery and Hormonal Therapy in Patients with Symptomatic Isthmocele
Figure 10. Pooled Analysis of Mean Duration of Menstruation Post Treatment Between Hysteroscopy and Vaginal Surgery in Patients with Symptomatic Isthmocele
Figure 11. Pooled Analysis of Intraoperative and Postoperative Outcome Between Hysteroscopy and Vaginal Surgery in Patients with Symptomatic Isthmocele
Figure 12. Pooled Analysis of Duration of Menstruation Post Treatment Between Laparoscopy and Hysteroscopy Surgery in Patients with Symptomatic Isthmocele
Figure 13. Pooled Analysis of Operative Outcome Between Laparoscopy and Hysteroscopy Surgery in Patients with Symptomatic Isthmocele