Authors / metadata
DOI: 10.36205/trocar4.2023001
Abstract
Introduction: Uterine leiomyomas are the most common benign gynecologic tumors afflicting women. Different types of minimal invasive gynecologic surgical procedures (MIGS) exist to treat leiomyomas. Tissue retrieval procedures came into disrepute since the Food and Drug (FDA) statements of 2014. The incidence of leiomyosarcoma in women is estimated to be 0,13 to 0,29 % whilst in patients undergoing power tissue retrieval an incidence of 1,2 % was observed. The aim of this prospective observational trial with retrospective analysis is to define the real incidence of leiomyosarcoma in women operated in Europe by MIGS.
Material and methods: From January 1998 through December 2004 in four Italian centers 2050 laparoscopic myomectomies for myomectomy or hysterectomy have been performed and two leiomyosarcomas have been found. One recognized and converted during surgery and one non diagnosed but recognized at frozen section. In a second phase from January 2005 to May 2015; 990 myomectomies -laparoscopic, laparotomic or hysteroscopic – and hysterectomies for myomas have been performed intwo centers (Italy and Belgium) using the International Society for Gynecologic Endoscopy (ISGE)preoperative screening protocol: clinical history, clinical examination, ultrasound scanning, blood testing.The low-risk patients to undergo the projected surgery and the high-risk patients to be screened bymagnetic resonance scan (MRI) before surgery if back to low risk by MRI these patients did undergo theprojected surgery. Other patients diagnosed as high risk for potential leiomyosarcoma were treated withan oncological regime.
Results: In total of 3040 myomectomies over a seventeen-year period in 6 centres in Europe (five in Italy and one in Belgium) have been performed. Three sarcomas have been diagnosed two in the first phase one during surgery and one immediately after surgery (frozen section). In the second phase one leiomyosarcoma has been detected using the ISGE recommendations prior to surgery. Detection rate of 0,10% or 1:1000 with one undetected (1:3040).
Conclusion: An improvement can be made in detecting leiomyosarcoma prior to surgery using preoperative history, blood testing (LDH) and imaging techniques, mainly vaginal ultrasound scanning and on indication MRI – the ISGE recommendations. In this study concerning patients undergoing MIGS, the incidence of leiomyosarcoma is of 0,10%. This is less than the estimated rate (0,13% – 0,29%) and much less than the rate found at power tissue retrieval (1,2%).
Introduction
Fibroids, also known as leiomyoma, fibro myoma or uterine fibroids are the most common benign tumours in women (1). Historically fibroids have been operated by laparotomy. At this day and age Minimal Invasive Gynecological Surgery (MIGS) including hysteroscopic myomectomy, laparoscopic myomectomy , with or without robotic approach, vaginal myomectomy, vNOTES myomectomy, with or without robotic techniques and hysterectomy performed by conventional laparoscopy or robotic-assisted, vaginal route or vNOTES are preferentially used. This shift to MIGS is explained by the reduction of the hospital stay, a reduced intra – and postoperative morbidity and a reduced delay to normal activity of the patient (2-12) In order to remove the fibroid or the uterus from the abdominal cavity some kind of volume reduction, to be able to remove the tissues, is mandatory. This can be achieved by power tissue retrieval (12). This technique does increase the risk of spreading both benign and malignant cell in the abdominal cavity and even in the MIGS entry wounds (13-25) The US Food and Drug Administration (FDA) communication on patients’ safety dated April 17th 2014 did change the approach of the surgeons to myoma surgery as it did urge them not to use tissue retrieval techniques based on power morcellation in treating the supposedly benign tumors. It did stress the concern that these techniques could be spreading cells of occult leiomyosarcomas and therefore reduce the survival rate of the patients (26,13-18,20-22). It also stated that samples obtained by power tissue retrieval could reveal to be difficult to interpret by pathologist due to the impact of the techniques on the specific tissues to be examined (26). This safety communication did lead to a cascade of statements by different entities warning against but not prohibiting power tissue retrieval Health Canada (27), the Society of Gynecologic Oncology (SGO) (28), the American College of Obstetricians and Gynecologists (ACOG) (29), the American Association of Gynecologic Laparoscopists (AAGL) (30), the European Society for Gynaecological Endoscopy (ESGE) (31), the European Society of Gynecological Oncology (ESGO) (32), the Asia-Pacific Association for Gynecologic Endoscopy and Minimally Invasive Therapy (APAGE) (33), the Australian Gynaecological Endoscopy and Surgery Society/The Royal Australian College of Obstetricians and Gynecologists (AGES/RANZCOG) (34), the British Society for Gynaecological Endoscopy (BSGE) (35), the French College of Obstetrics and Gynecology (36), and the German Society for Gynecology and Obstetrics (DGGG) (37). The FDA analysis went out from the estimate that 1/350 women undergoing hysterectomy for myoma could have an occult leiomyosarcoma this in contrast to the findings of the American Cancer Society estimating that only 1600 cases of newly diagnosed uterine cancers in 52.000 registrations reveals to be a uterine leiomyosarcoma (38). On top of these FDA warnings Seidman et al (2012) did observe that in 1,2% of the power tissue retrieval cases unexpected diagnoses of atypical or malignant smooth muscle had been made, in cases diagnosed preoperative as benign fibroids (39). In a prospective study Sizzi et al did find one case in 2050 myoma tissue retrievals (0.04%) (40). In its 2021 statement the Italian Association of Medical Oncology (AIOM) stated for the impact to be of < 2/100000 women/year with an average age of detection at 56 years (41). In view of the above data the overall risk of an occult uterine leiomyosarcoma appears low. However, patients needing to undergo a MIGS and require intracorporeal tissue retrieval are in need of an appropriated preoperative evaluation to exclude a potential occult malignancy. The aim of this study is to prove that the ISGE guidelines do offer the clinician a workable flowchart to discuss the preoperative findings with the patients as to offer a workable solution for both patient and surgeon (42). This by confronting the data of a prospective Italian observational study, reanalyzed retrospectively with a prospective observational study using the ISGE recommendations as a preoperative diagnostic flow chart (40,42).
Methods
The results of the first prospective observational study have been reevaluated looking at the presence of leiomyosarcomas. In the second prospective observational study patients needing a myomectomy, for clinical symptoms or fertility indications, or a hysterectomy for symptoms were subjected to the ISGE recommendations as follows: a first consultation was held with the individual patient where the different ISGE guidelines were explained in detail and the patient did sing a consent form after understanding and agreeing with the proposed guidelines. Patients who did not sign the consent from were excluded from this study. As this last study was a prospective observational study dealing with normal surgical interventions and the patients did give their consent prior to the study the personal data do resort under the privacy, later GDPR, regulations and ethical approval was not asked for (43,44).
Clinical exam considerations
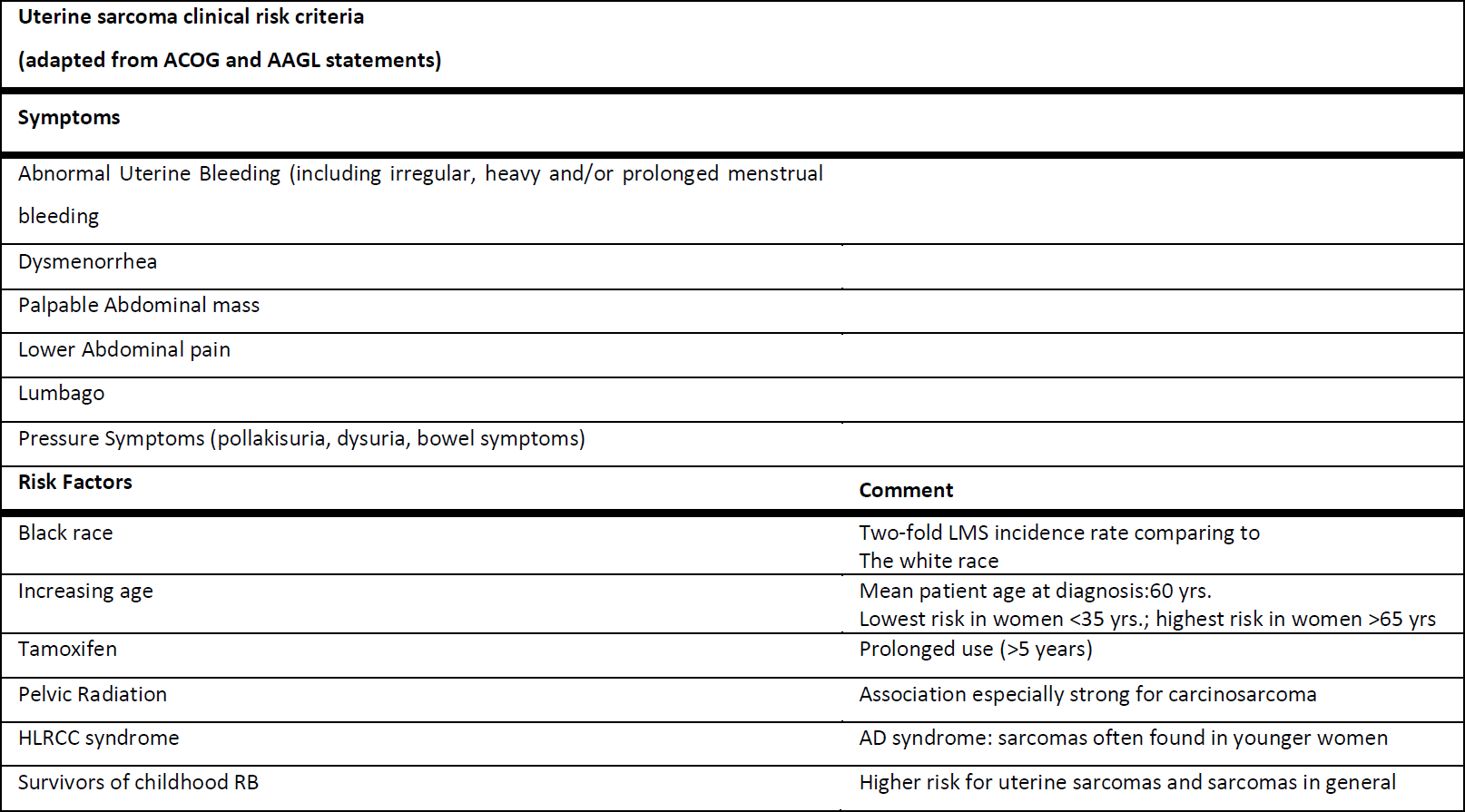
All patients needing a myomectomy of hysterectomy with MIGS did undergo a structured interview concerning their medical history. Projected against the known leiomyosarcoma risks (Table 1) Black race is a common risk factor for both LM and LMS. In black vs. white women, the LM incidence is 2- to 3-fold greater and the LMS incidence as well as the incidence of carcinosarcoma is 2- fold higher (45-48). In contrast, increasing age, particularly postmenopausal status, is an important risk factor for uterine sarcomas, while LM typically shrinks following menopause. Below the age of 40, sarcoma in a presumed LM is extremely rare (31). Tamoxifen used for five years or more, pelvic irradiation, hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome due to germline mutations of fumarate hydratase, and a history of childhood retinoblastoma constitute other risk factors for uterine sarcoma, which do not present statistically significant association with LM. The clinical manifestations of LM and LMS are often indistinguishable (29,30). Abnormal uterine bleeding (AUB) (most frequently, in the form of heavy menstrual bleeding), dysmenorrhea, lower abdominal pain, lumbago, pressure symptoms (e.g. pollakisuria, dysuria, bowel symptoms) and palpable mass on the site of lower abdomen are symptoms and signs for both LM and LMS (29,49–52). Uterus and lesion size, uterine contour, mobility and any other examination finding cannot accurately distinguish a LM from a LMS (49,50,53-55). Rapid lesion growth has been traditionally valorized as a sign of a potential malignancy (56) however, neither large tumors (49,54) nor rapidly enlarging uterine masses (53,55,57–60) have been demonstrated as useful malignancy indicators in premenopausal women. On the other hand, in postmenopausal patients, particularly in women who are not on hormone replacement therapy, new or growing lesions require evaluation and malignant process exclusion. Furthermore, the lesion failure to respond to medical or non-excisional treatment, such as uterine artery embolization or myolysis performed by magnetic resonance-guided focused ultrasound (MRgFUS), is clinically highly important, despite the fact that it does not provide absolute evidence of malignant nature (61–67). The metastatic disease manifestations can be found in women with LMS, while spontaneous benign LM dissemination is very rare, except when power tissue retrieval methods are used, giving origin to disseminated peritoneal leiomyomatosis, intravascular leiomyomatosis and benign LM metastasizing to distant tissues (68).
The following initial exam does include a physical examination and a routine screening for cervical cancer
Routine screening for cervical cancer and endometrial sampling
Prior to treatment of presumed LM, routine screening for cervical cancer should be performed. Uterus morcellation should be avoided in women with cervical dysplasia (30). To rule out endometrial atypical hyperplasia or endometrial cancer in women with abnormal uterine bleeding, most guidelines suggest that patients should be selected for endometrial sampling based on a combination of the factors indicating an increased risk (e.g. patient’s age, genetic and personal risk factors, endometrial echo-features) (69). Although the results of previous smaller studies (70,71), show that the sensitivity of endometrial biopsy to detect LMS is low, positive or suspicious results do have decisive impact on management of that individual patient (72).
Biochemical markers
Patients with LMS often have somewhat increased serum lactate dehydrogenase (LDH) levels (73) especially isoenzyme 3 (LDH3) (74). For instance, Goto et al. observed an abnormally increased level of total LDH and LDH3 in all patients with LMS (LDH3, sensitivity and specificity 90% and 92.3%) (74). These studies have not been reproduced. Elevated serum cancer antigen 125 (CA125) has been occasionally observed in LMS in advanced-stage disease (75,76). However, Menczer et al. did not evidence CA125 expression in any of 17 immunohistochemically examined LMS tumors, concluding that the origin of increased serum CA125 is not in neoplastic tissue (77) As there is significant overlapping levels of CA 125 between LM and early LMS this biochemical marker is of no use in a flowchart to detect LMS preoperatively (75).
Imaging: Ultrasonography
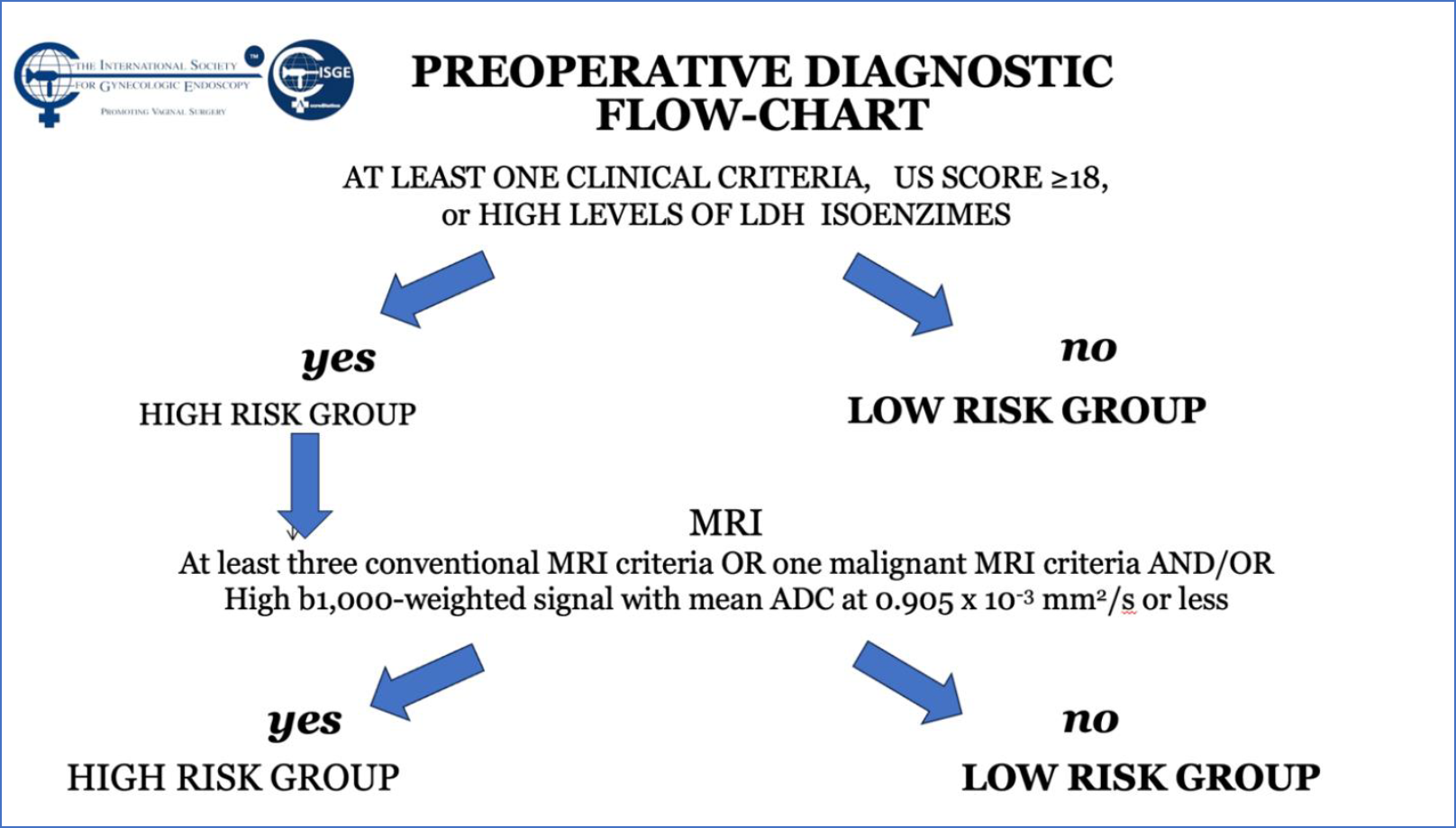
The vast majority of fibroids are discovered and evaluated via ultrasound scanning, using the trans-abdominal and transvaginal routes, due to its accessibility, relatively low costs and reliability (1). The Morphological Uterus Sonographic Assessment (MUSA) consensus paper, published in 2015, provides the terms, definitions and measurements for standardized evaluation and reporting of sonographic features of the myometrium and myometrial lesions (78). Sonographically, a typical uterine LM is a well-defined round formation within or attached to the myometrium, its echogenicity varies, often showing some internal hyper echogenicity, internal fan-shaped shadows and/or shadows at the edge of the lesion (78). Circumferential flow around the formation can be visualized by color or power Doppler, the MUSA group suggests that LM should be labeled as sonographically atypical if the lesion does not exhibit this vascular pattern. When a LM degenerates, it may show low echogenicity, a hyperechogenic rim and no internal vascularity, or mixed echogenicity, or hypoechogenic cystic areas (78 -81). LM with little or no recurrent and/or metastatic potential as well as uterine Smooth-muscle Tumors of Uncertain Malignant Potential (STUMP) have the same ultrasound characteristics as does an ordinary LM (78,82–87). Several studies focused their attention on the detection of sonographic parameters that could be used in distinguishing between a benign and a malignant uterine smooth muscle tumor (89-92) Uterine sarcomas are typically solitary, large lesions often exhibiting ultrasound features that are indistinct from those of LM (81,92, 93). Although they may also appear as irregularly and highly vascularized masses, with or without irregular anechogenic areas reflecting tumor central necrosis (1,88,91,94,95), there is no ultrasound characteristic that can reliably differentiate between LM and LMS. Exacoustos et al. proposed a subjective semi-quantitative assessment of the blood flow (vascular score), examined with directional power Doppler imaging, which was similar to that proposed for adnexal masses by the International Ovarian Tumor Analysis (IOTA) Consensus Group, revealing that the increased central and peripheral vascularity had a sensitivity, specificity, and positive predictive value (PPV) of 100%, 86%, and 19% in the diagnosis of LMS (94). However, a 19% PPV is not clinically relevant. This being the reason why the ISGE did propose a scoring system for the ultrasound evaluation of the LM with a cut off at score of 18 points (Table 2) (Figure 1). This guiding the physician to evaluate the individual situation in the by ISGE proposed flowchart (Table 3) (42).
Imaging: Magnetic resonance imaging (MRI)
MRI provides a better image in delineating the exact location and characteristic of the fibroids; the down point is that this exam it is far more expensive and less accessible than ultrasound (1,89). Therefore, MRI should be considered only for women in whom the nature of the pelvic mass is uncertain after clinical and pelvic ultrasound, preferably vaginal ultrasound, assessment as recommended by the ISGE guidelines if the score is higher or equal to 18 points (Fig 1). The differentiation of LM from LMS may be suggested by assessing tumor total necrosis and the presence of a peripheral rim, which corresponds to the obstructed veins showing low signal intensity on T2 and high signal intensity on T1WI (96). Tanaka et al. reported that the highest accuracy in diagnosing non benign smooth muscle lesions is expected when more than 50% of the tumor shows high signal on T2WI (97). Goto et al. compared conventional MRI findings along with post-enhancement behavior (dynamic MRI) of degenerated LM and LMS, revealing a specificity, accuracy and positive predictive value (PPV) of 96.9%, 97.1%, and 71.4% for MRI, and 87.5%, 90.5% and 71.4% for dynamic MRI (74). Both sensitivity and negative predictive value (NPV) were 100%. Sato et al. proposed the use of Diffusion-Weighted Imaging (DWI) and corresponding Apparent Diffusion Coefficient (ADC) values in the evaluation of myometrial tumors (98). DWI provides quantitative measurements for the ADC -ADC being a mathematic mapping not a realimage – and these are considered to beinfluenced by nuclear to cytoplasm ratio andcellular density in solid tissues. Both DWI andADC therefore do need a T2 image of thestructure. Sato et al. affirmed that cases withlow signal intensity on DWI may be regarded asuterine LM, while intermediate to high signalintensity may indicate uterine LMS. For thisreason, in patients with parenchymal areas ofintermediate to high signal intensity, ADC valueswere evaluated, revealing that the mean ADCvalue for the LMS lesions was significantly lowerthan that of the LM nodules (98). Neoplasms arecharacterized with high cellularity this reducesthe free motion of water molecules on DWIimaging increasing the signal. On ADC MAPs thesignal of these areas is hypointense (99).
Results
From the first prospective study after retrospective analysis of 2050 patients two LMS have been diagnosed at operation one and one immediately after surgery during the operation itself by frozen section and in both cases the operation has been converted to an oncological procedure.
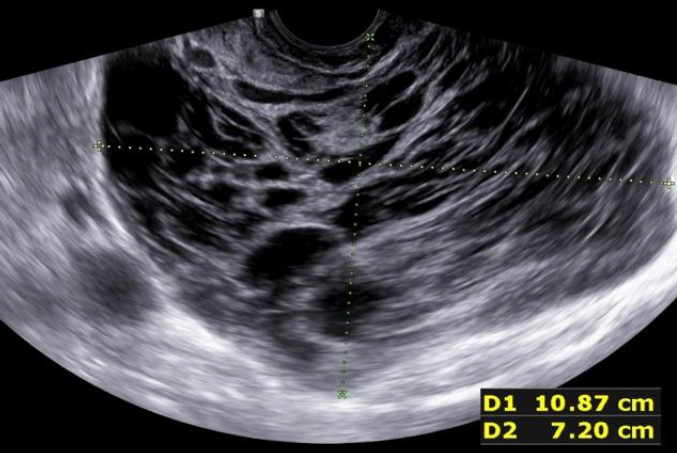
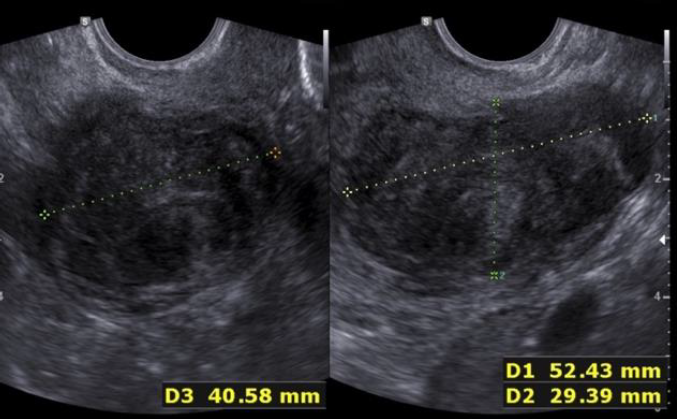
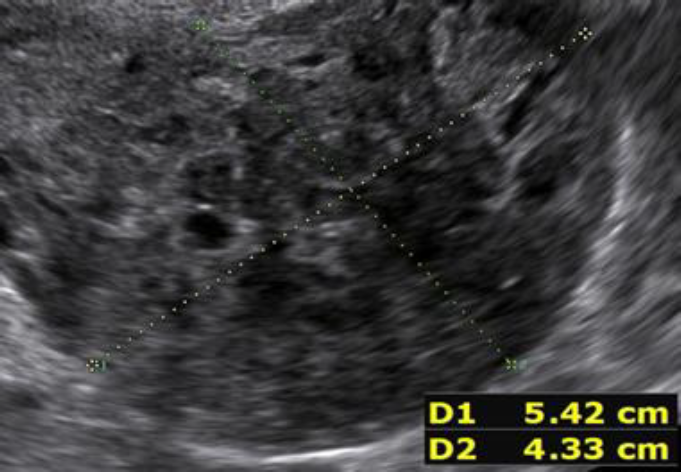
During the second prospective observational study the ISGE recommendation: 1. Structured interview taking into account the different common risk factors of LMS (table 1) 2. Structured clinic clinical exam with exclusion of cervical malignancy and if needed intrauterine malignancy indicators 3. Vaginal ultrasound evaluation in accordance with the ISGE recommended scoring system for ultrasound (table 2, figure 1) and finally adherence on the ISGE flowchart (table 3) have been followed in 990 patients. One LMS was detected before surgery with as characteristics: patient 52 years of age, solitary lesion of 10.87 x 7;20 x 8.0 cm – large – ultrasound evaluation score 22/25 (figure 2, 7, 11). On ISGE flowchart HIGH RISK. One other patient was detected preoperatively as a Uterine Smooth-muscle Tumor of Uncertain Malignant Potential (STUMP). The patient of 52 years of age presented with a solitary lesion of 52.43 x 29.39x 4.58 cm with rapid increase of volume with strong inhomogeneous echotexture without cystic degeneration. The ultrasound score was 19/25. ISGE flowchart HIGH RISK (figure 3,10). A third patient, 32 years of age, was first considered to be possible LMS preoperatively but averred on histology to present an intramural leiomyoma with hyper cellular areas, focal sclerohyalinosis and fatty degeneration. Her ultrasound score was 20/25 (fig 4, 9). On ISGE flowchart initially HIGH RISK but converted after MRI to low risk. The final histopathology in the last patient was atypical leiomyoma with a high mitotic index. Three other patients age range between 38 and 43 presented with normal LDH and ISGE scores between 22 and 19 al these patients were cleared by MRI as LOW Risk in all cases pathology did detect less than 10 mitoses per high power field. As all ultrasound scores were over > 18 all patients were confirmed by MRI. None of the other 987 patients had an abnormal pathology report (ultrasound score </= 18).
Discussion
In this study concerning patients undergoing MIGS, the incidence of LMS is of 0,10%. This is less than the estimated rate of (0,13% – 0,29%) in the literature and much less than the rate found at power tissue retrieval (1,2%). The conclusion from these numbers therefore is that some data in literature are overestimated and that the true incidence of LMS is between 0,10% and 0,29%. However, it is important to state that the final diagnosis of LMS can only be reached through the lesion’s histological evaluation. From the numbers of the above prospective trial, it is shown that starting in patients over 30 years of age more mitoses per high power filed at pathology are found. Further it is essential to realize that the incision in the uterine serosa and myometrium, necessary to extract LM, by definition opens vascular and lymphatic channels, facilitating the spread of tumor cells if dealing with LMS. Any blood or tissue fragments spilled into the abdominal cavity as well as contact of any resected tissue with the peritoneum can potentially spread cancerous cells. By the time the tissue has been placed in a bag for morcellation, it may be already too late to prevent the spread of tumor (100,101). On the other hand, it could be considered to perform total laparoscopic hysterectomies instead of supra-cervical hysterectomies, with tissue retrieval of the specimen through the vagina, in a bag. There are no evidence-based medicine data showing that supra-cervical hysterectomy is better for the patient on the long run compared to total laparoscopic hysterectomy (102). Preference to supra-cervical hysterectomy can be justified only in case of pelvic organ prolapse repair after appropriate preoperative evaluation. Currently, there is not a single tissue-extracting procedure that offers absolute patient protection. Thus, all methods should remain available (30). In all circumstances when preoperative evaluation of cervix, endometrium and/or myometrium results in an increased suspicion for malignancy, the surgeons should employ alternatives to tissue retrieval, including laparotomy (29–31,38). Inversely, low-risk patients may undergo and experience benefits from minimally invasive surgery avoiding per- and postoperative complications (103).
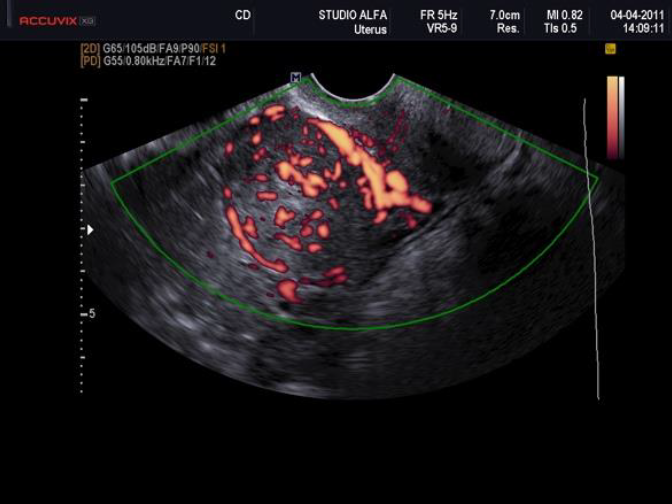
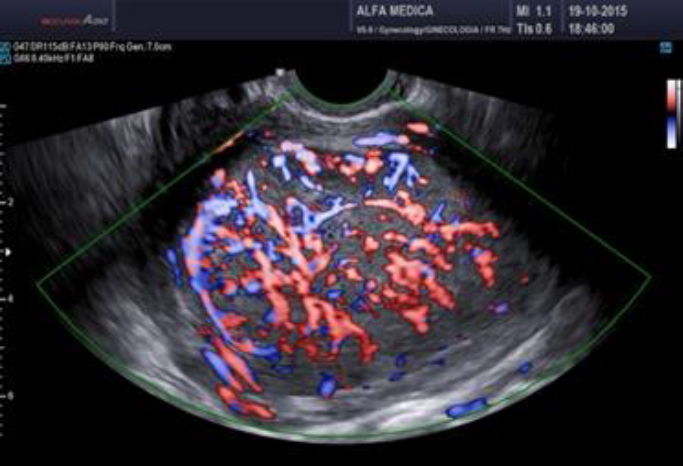
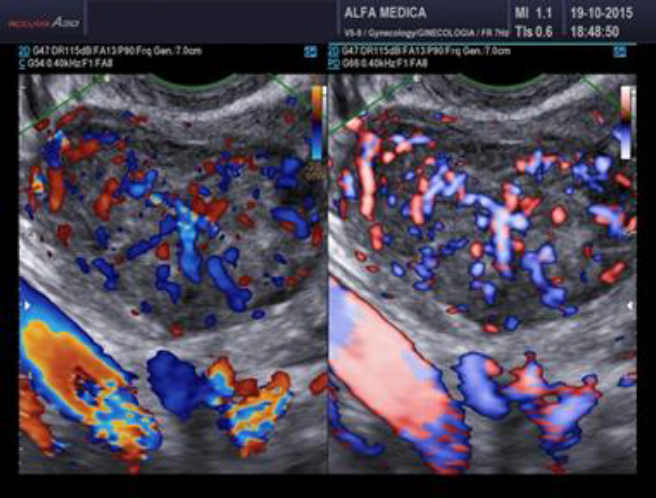
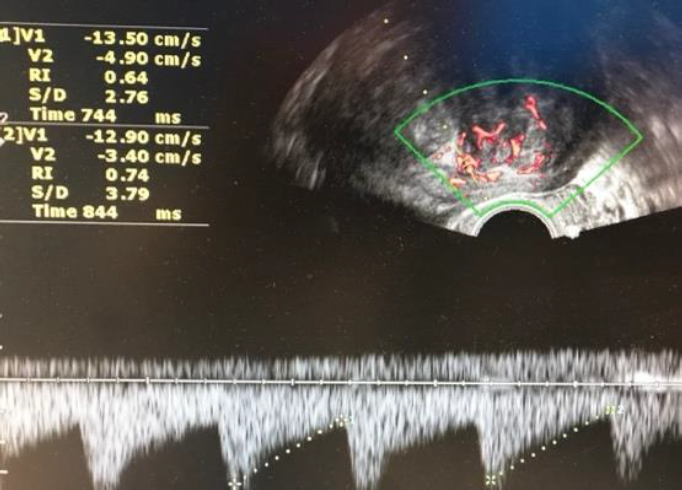
Surgeons and hospitals should always be aware that sarcomas are diagnosed, although very rarely, even in patients appropriately evaluated and selected for tissue retrieval-involving interventions (104,105). A new aspect of ultrasound is elastography. In the future this technique of reporting stiffness in tissues could be integrated into to preoperative ISGE guidelines (106). As MRI is not always easily accessible and available it should be reserved for those patients who are classified high risk after ultrasound scanning. Dealing with MRI it is important to consider the T2 image so that the DWI can be interpreted and the ADC can be mathematically constructed. It is the ADC mapped image that is the most reliable to diagnose LMS. ISGE does advise that the ultrasound scanning is made by the vaginal approach as to be as close as possible to the target tissue. Power Doppler enhanced vaginal ultrasound scanning helps to define the peripheral vascularization in LM (figure 5). It does also help to identify the internal vascularization (figure 6,7). Measurement of the Resistance Index (RI) of the vascular flow can in some cases help (fig8). ISGE also advises for the gynaecologist to perform the vaginal ultrasound examination her or himself or at least to closely observe the examination as to apply the scoring system him or herself. The use of the preoperative ISGE guidelines especially the ISGE flowchart seems to offer the individual physician tools to enable individualizing patients at risk for LMS.
References
Table 1: known leiomyosarcoma risk
Table 3: ISGE Flowchart to distinguish LM from LMS
Fig. 1: Practical scoring system of ultrasound features and age data
Fig. 2: Prospective study 2 Conventional ultrasound scanning Leiomyosarcoma detected preoperatively. US Score 22/25
Fig. 3: Patient in phase two prospective trial Conventional ultrasound scanning detected with STUMP US score 19/25
Fig. 4: 32-year-old patient in phase two prospective trial: Conventional ultrasound scanning with Intramural leiomyoma with hypercellular areas, focal sclerohyalinosis and fatty degeneration. US score 20/25
Fig. 5: Example of peripheral vasculature Power Doppler
Fig. 6: LMS with marked peripheral and internal vasculature
Fig. 7: LMS Note the heavy doppler signals not around the mass but also inside. (Doppler (left) and Power Doppler (right) intensities)
Fig. 8: LM: Normal peripheral vasculature at Doppler sometimesthe Resistance index (RI) can help out. Normal curves and RI of the vessels 0.74 (cut off for malignancy RI 0.45)
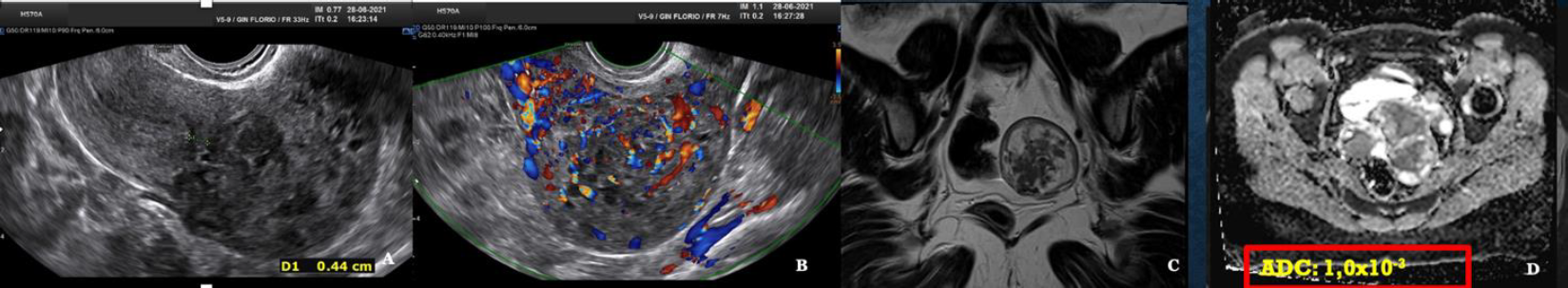
Fig. 9: Atypical myoma with high mitotic activity. A vaginal ultrasound greyscale. B Vascular halo but also internal vascularization. C T2 MRI. D ADC mapping with measurements in the high range. ADC 1,0×10-3
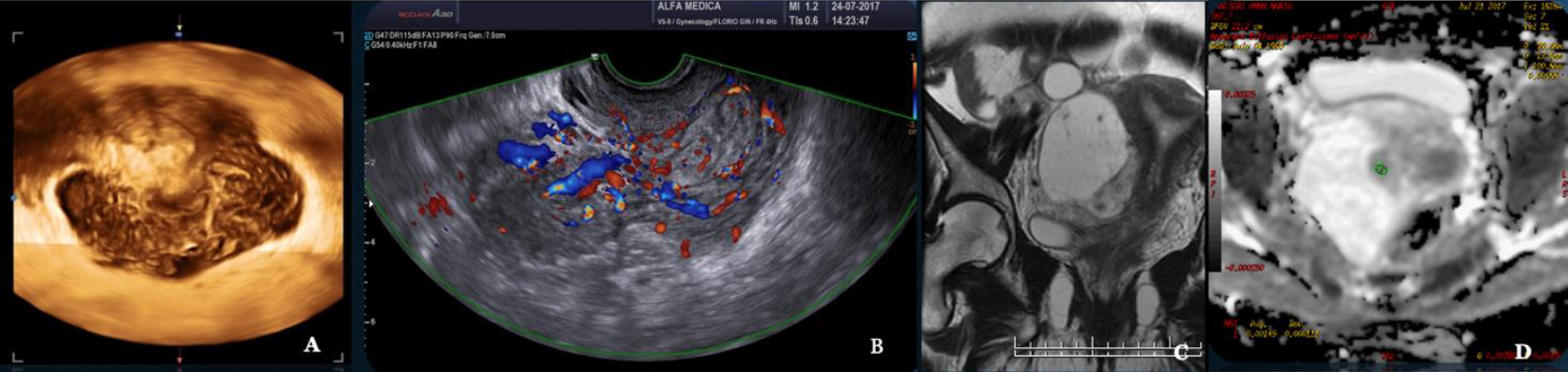
Fig. 10: Smooth muscle Tumor with Undefined Malignant Potential (STUMP) A Three D vaginal scanning B Doppler Ultrasound ISGE US score 22/25 C MRI T2 image. C ADC Mapping ADC in the high range: 1,1×10-3
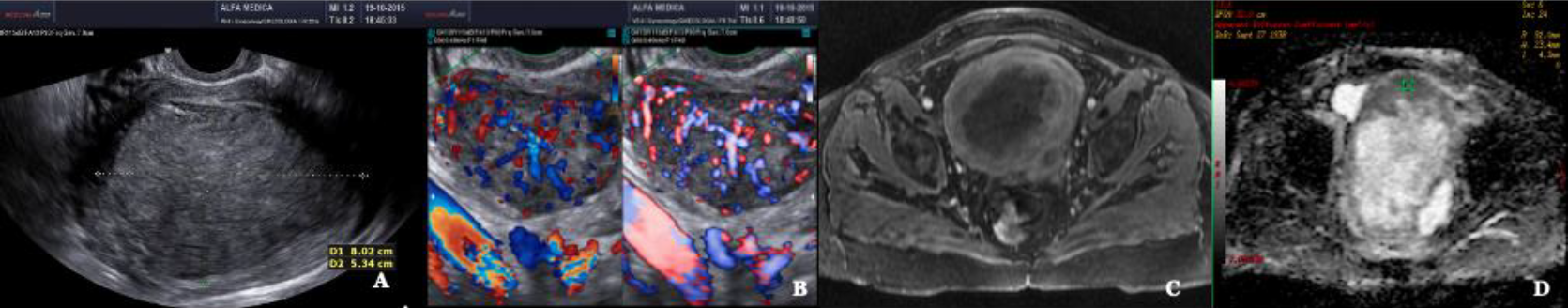
3Fig. 11: Leiomyosarcoma A Conventional ultrasound scanning B Doppler enhanced ultrasound scanning (left) Power Doppler enhanced ultrasound scanning (right) C MRI T2 image D MRI ADC mapping ADC in the lower range: 0,9×10-