Authors / metadata
DOI : 10.36205/trocar3.2023002
Abstract
Objective: Recurrent Implantation Failure (RIF) remains the most challenging in-vitro fertilization (IVF) problem to treat. This is because the overall success rate is only approximately 30%. Hysteroscopy remains the gold standard for diagnosing and treating intra-uterine anomalies. This study aimed to evaluate the role of hysteroscopy (HSC) in improving pregnancy outcomes in patients with RIF.
Methods: A systematic search was performed in PubMed, ScienceDirect, Embase, and Cochrane using MeSH terms, if applicable and in accordance with the PRISMA guidelines, to determine the role of hysteroscopy compared to patients who didn’t undergo hysteroscopy. The Newcastle– Ottawa scale (NOS) was used to assess the risk of bias in this analysis, and Review Manager
5.4 was used to calculate the result of 95% CI for the outcomes. The endpoints of interest were clinical pregnancy rate, live birth rate, implantation rate, and miscarriage.
Results: A total of 3 randomized controlled trials (RCT) and 5 cohort studies with 4,679 patients were included. Pooled analysis showed that patients who underwent HSC were associated with higher clinical pregnancy – [OR 1.64, 95%CI (1.32-2.03)], live birth – [OR 1.50, 95%CI (1.17-1.92)], and implantation rate [OR 1.42, 95%CI (1.02-1.98)] but no significance in miscarriage rate. Further subgroup analysis suggests HSC had a significantly greater effect on clinical pregnancy rate for patients with abnormal HSC findings [OR 1.20, 95%CI (1.01-1.42)], but no significant difference in live birth – and miscarriage rate.
Conclusion: HSC plays a significant role in improving the clinical pregnancy rate, especially in patients with abnormal HSC findings. HSC also improves implantation rate, live birth -, and clinical pregnancy rates in patients with RIF. Since the number of the study is still limited, further investigations are still needed to confirm the results.
Introduction
Infertility is a major issue that affects millions of couples worldwide. In the United States, around 7.5 million couples, or 1 in 8, are affected by this condition (1). The situation is not any better in Indonesia, where a study revealed that 21.3% of couples have trouble conceiving or sustaining a pregnancy, affecting roughly one in every five couples (2).
Fortunately, a solution to this problem is assisted reproductive technology (ART). Among the most frequently techniques used in ART is in vitro fertilization (IVF). Studies have shown that IVF is an excellent solution for treating infertility (3-5). It is crucial to understand that the success rate of IVF cycles resulting in live births is approximately 25-30%. Among the numerous obstacles encountered during IVF, treating recurrent implantation failure (RIF) represents the most formidable challenge due to its low success rates of around 30% for women with RIF. While ovum collection and fertilization are often successful, an inexplicable discrepancy exists between the number of embryo transfers and the number of ongoing pregnancies lasting over 12 weeks (6).
The reason for this failure to implant is not yet fully comprehended, although it appears to be influenced by both the embryo itself and the uterine cavity (7,8). Some abnormalities in the uterine cavity, such as polyps, myoma, adhesions, and sometimes endometriosis, are thought to be associated with impaired implantation and reduced chance of pregnancy (9). Several studies have reported the influence of intrauterine pathologies on pregnancy rates in women who will undergo IVF (10). Therefore, it is advisable to perform an examination for intrauterine pathologies before starting IVF (11,12).
Since hysteroscopy (HSC) can give a good view of the uterine cavity, it is regarded as the reference standard for detecting these uterine abnormalities (13,14). HSC are reported to significantly find more abnormalities in patients with a history of ART failure (15-17). Two randomized controlled trials (RCT) confirmed the value of HSC in women with RIF by showing an increase in clinical pregnancy rate as high as 13%. In clinical practice, hysteroscopy is often performed on infertile women scheduled for the first IVF cycle. However, several studies have shown no significant effect of routine HSC on live birth rates (17-19). Due to conflicting findings regarding the use of HSC in patients with RIF, this study was aimed to determine if HSC before starting an IVF cycle in women with RIF may improve the clinical pregnancy rate, implantation rate, and live birth rate, this study was also aimed to see whether HSC reduces the miscarriage rate in IVF patients.
Materials and methods
Systematic Reviews and Meta-Analyses (PRISMA) (20). This research collects and uses previously published studies. Therefore, there is no need for ethical approval. The submitted protocol was registered on the International prospective register of systematic reviews-PROSPERO (www.crd.york.ac.uk/prospero).
Search strategy and selection criteria
Medical Literature Analysis and Retrieval System Online (MEDLINE) via PubMed, EMBASE (Excerpta Medical Database), Science Direct and the Cochrane Library were searched without any language restriction from January 2002 until February 2023, using the following keywords: ‘in vitro fertilization’ or ‘in-vitro fertilization’ and ‘infertility’ and ‘hysteroscopy’ and ‘recurrent implantation failure’ or ‘embryo implantation’ or ‘treatment failure’ and ‘uterine disease’ and ‘pregnancy’. Citation tracking was performed to identify additional publications. Our study searching protocols are presented in Supplementary Table S1.
All identified studies were screened by title and abstract. The inclusion criteria in this study were randomized controlled trial studies, non-randomized two-arm prospective studies, and two-arm retrospective studies. The study population was women with normal ultrasound examination of the uterine cavity and women who had recurrent implantation failure, defined in this study with at least 2 failed IVF embryo transfer attempts. Before starting IVF cycles, patients underwent HSC diagnostic. Meanwhile, the control population did not have a HSC before starting IVF. On the other hand, the exclusion criteria in this study were one-arm studies, article reviews, case reports, proceedings, and personal comments, studies with no data of outcome interest, and studies that aimed to assess the efficacy of HSC-associated scratching, biopsy, or treatment were also excluded. Two investigators independently identified studies that met the inclusion criteria, and the third investigator was consulted on whether any disagreements or to resolve any differences. A discussion was conducted to make the final decision.
Data extraction; quality assessment
Data extraction and quality assessment were carried out independently by two investigators. Standard forms were used to extract the following information from each study: the study authors; study design and methodology; total and mean age of the patients; intervention used for the patients; IVF cycles failed; definition of RIF; clinical pregnancy rate; live birth rate; miscarriage rate; and implantation rate. In cases of missing data in the main results or something unclear, the authors of the original publication were contacted via email.
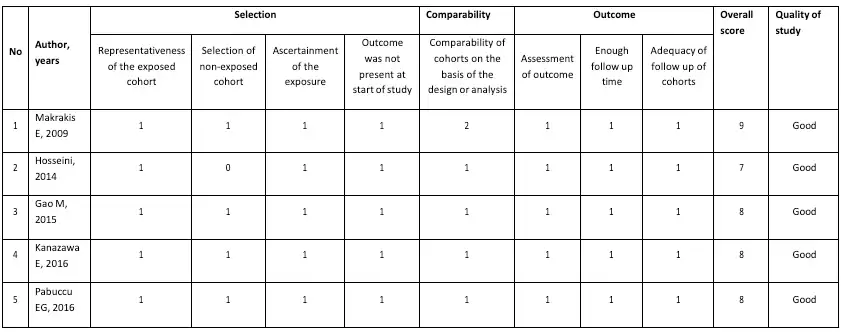
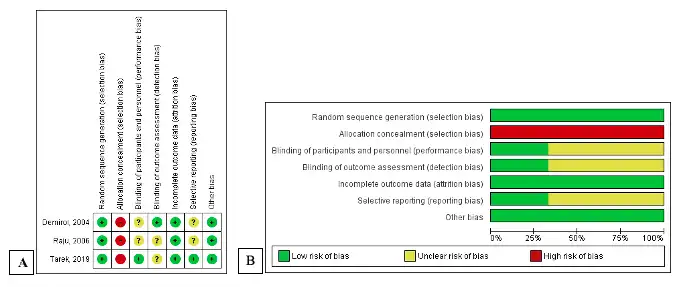
The risk of bias assessment for the included studies was conducted based on the study type. The randomized control trial study (RCT) was assessed using the Cochrane Risk of Bias tool (RoB) (21). The RoB consists of seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Other sources of bias included potential bias related to the specific study design, stopped early due to some data-dependent process, and extreme baseline imbalance. The information extracted from the paper was judged on the possible risk of bias in each domain and was rated as “low risk,” “unclear,” or “high risk.” For non-randomized studies, the risk of bias was analyzed by the Modified Newcastle-Ottawa Scale for Cohort Studies (22). The scale contains eight items within three domains. The possible total point for domain selection is 4 points, 2 points for comparability, and 3 points for outcome domain. The quality of the study was classified as “good” if the total was 7-9, “moderate” if the total score was 4-6, and otherwise as “poor.” Two reviewers independently conducted the risk bias assessment, and any disagreement was resolved by discussion with the third reviewer. The overall quality of the non-randomized studies was good and presented in Table 1 as the risk of bias individually. The summary of RCTs quality is shown in Figure 1.
Outcome measurement
This study aimed to see whether there is any role for hysteroscopy in patients with recurrent implantation failure before starting in vitro fertilization considering clinical pregnancy rate, live birth rate, implantation rate, and miscarriage rate. Clinical pregnancy was defined as thirty-five days after embryo transfer and ultrasound examination showing a gestational sac, live birth rate was defined as the number of deliveries that resulted in a live-born neonate, and implantation rate was defined as the number of gestational sacs determined by ultrasound by the number of embryos transferred.
Data Synthesis and Analysis Quality Assessment; the meta-analysis was performed using Review Manager 5.4. The risks in terms of the outcomes of interest were computed and will be processed using Review Manager 5.4 and will later be presented with odds ratios (ORs) with 95% confidence intervals (CIs). Heterogeneity analysis between study populations was calculated using the I2 statistic. The I2 statistic was defined as follows: 0-24% as no heterogeneity, 25%-49% as moderate heterogeneity, 50-74% as considerable heterogeneity, and 75%-100% as extreme heterogeneity (23). Data are summarized across groups using the Mantel-Haenszel (M-H) risk ratio (RR) fixed effect model if I2 < 25%. The random effect model is used if I2 > 25% (24). Funnel plots were used to evaluate publication bias. Analysis was carried out using Review Manager 5.4.
Results
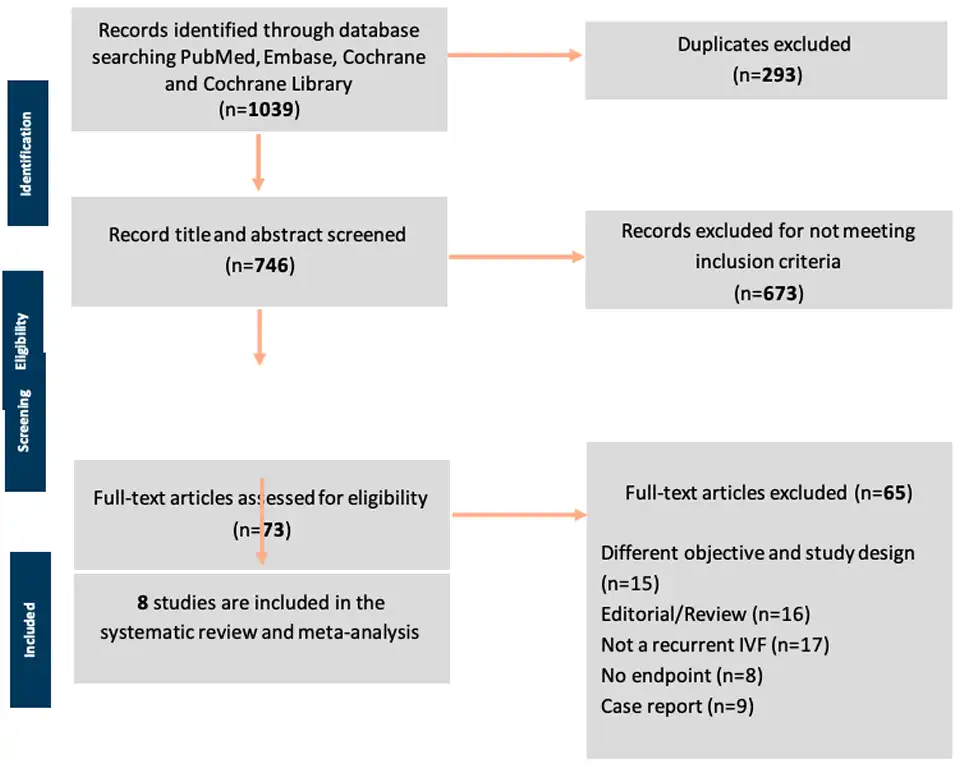
Literature Search: The flow diagram of the study selection process is shown in Figure 2. A total of 1039 studies were found during the initial screening through database searching and other sources. Two hundred ninety-three studies were removed due to duplicates, leaving 746 studies. These were scrutinized further for title and abstract and 673 studies that did not meet the inclusion criteria were excluded. The remaining 73 full-text articles were finally reviewed. As many as 65 studies were excluded due to different objectives and study designs (n=15), review articles (n=16), not a recurrent IVF but rather the first IVF cycle studies (n=17); no endpoints or different outcomes of interest (n=8); and case report studies (n=9). Finally, only eight studies were included in the meta- analysis (25 – 32)
Characteristics of included studies
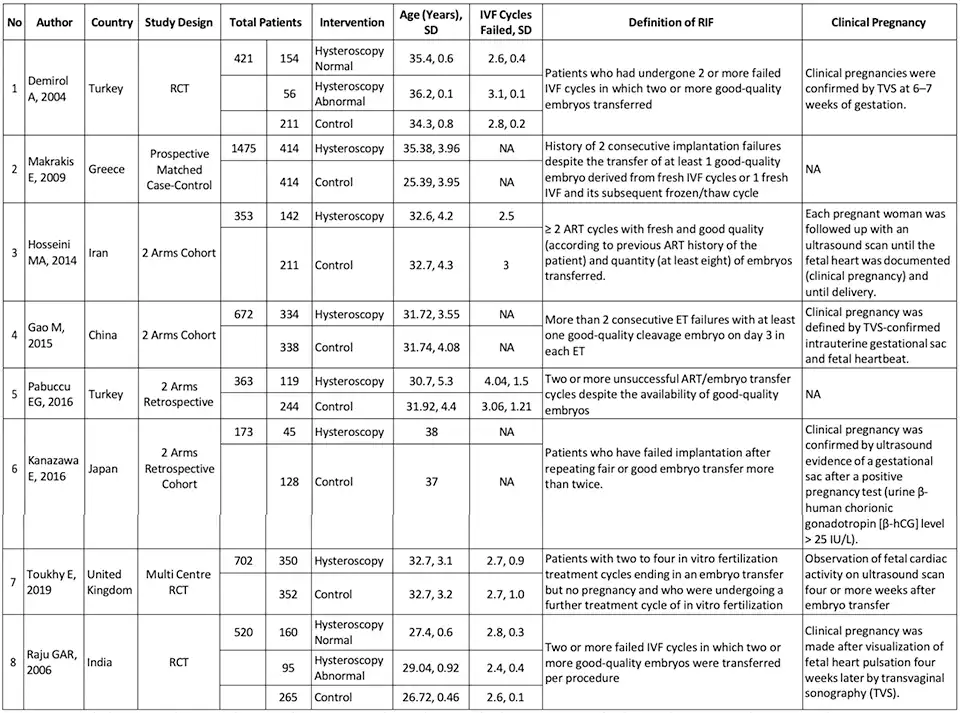
In this meta-analysis, eight studies met the predetermined inclusion criteria comprising three RCT studies, two retrospective studies, and three cohort studies. The basic summary of study characteristics included in this review, study design, the total of patients and the percentage of patients forming the population of each intervention, mean age, mean failed IVF cycles, the definition of RIF, and clinical pregnancy definition from each of the studies are represented in Table 2.
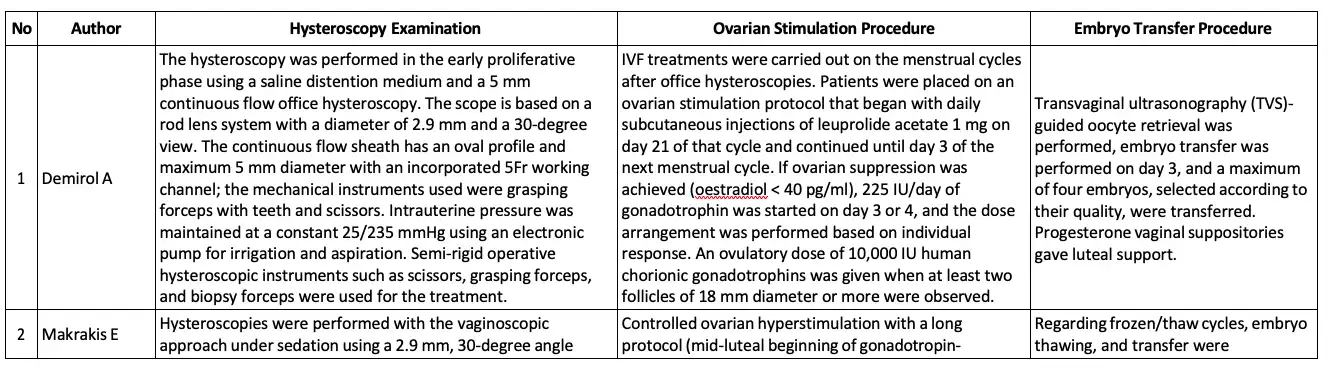
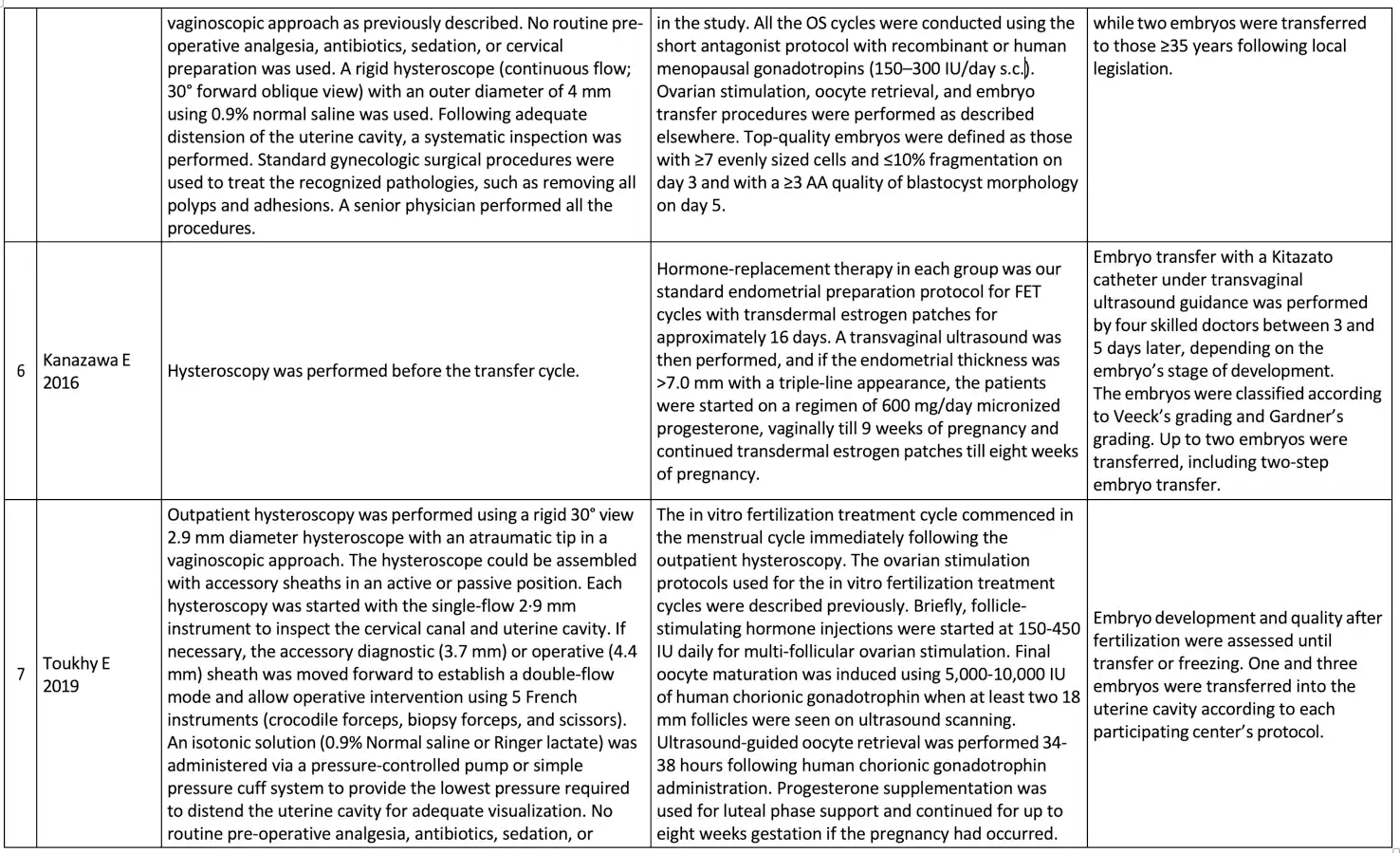
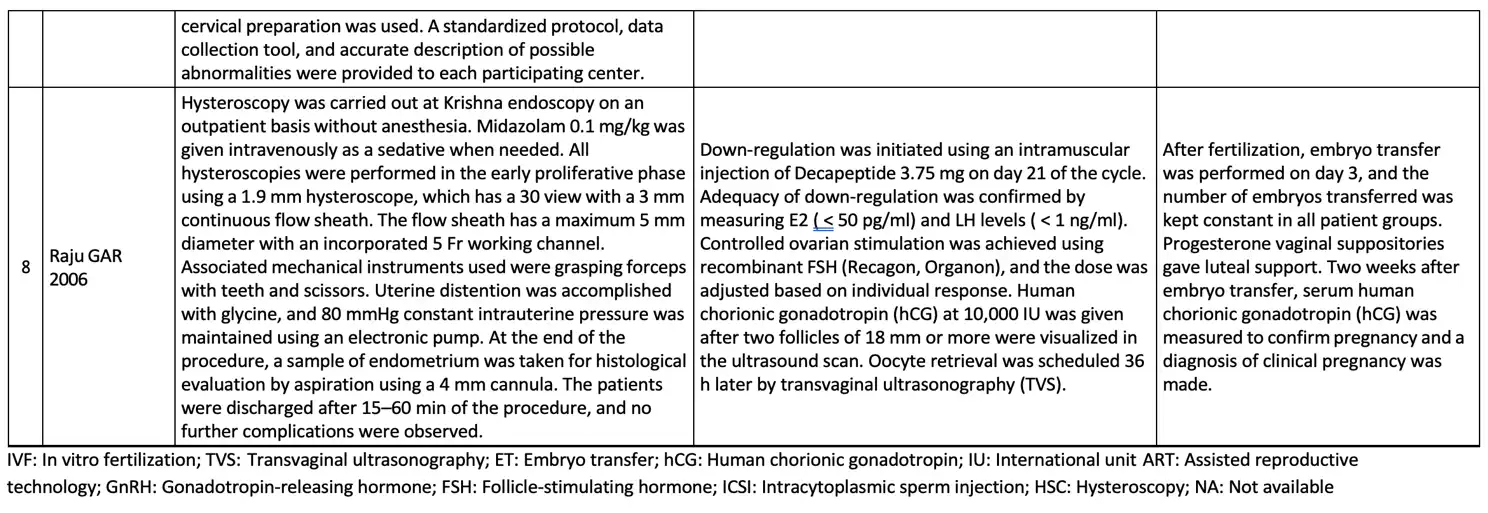
The basic summary data concerning the hysteroscopic examination procedure, ovarian stimulation procedure, and embryo transfer procedure is available in Table 3.
There are three studies with a RCT design. All of the studies did use computer- generated randomized systems, therefore these are rated as studies having a low risk of bias. Tarek et al., and Raju et al., needed clarification about the blinding outcome assessment domain because there was no statement about blinding the assessor. Similarly, in the study by Raju et al. and Demirol et al., there is insufficient data for the blinding participant and personnel domain to declare the risk of bias. As bias due to allocation concealment, all studies were considered high risk. The hysteroscopic procedure was explicitly unconcealed, which cannot be masked between the control and experiment groups. Tarek et al. published a study protocol that explained clearly the study outcome, thus getting a low risk of selective reporting. Meanwhile, the study protocol for the rest of the studies were unavailable.
All cohort studies were of quality, with a score of 7-9. The analysis by Hosseini et al. made no point in selecting a non-exposed cohort due to the fact of using an historical cohort as control compared to the present cohort, which means that the control cohort group did not resort from the same population The excellent quality in the selection domain must consist of inclusion and exclusion criteria to ascertain the representativeness of the cohort, pick the non-exposed group from the same cohort, have a good record of exposure, and ensure no outcome is present at the start of the study. Except for Hosseini et al., the rest of the studies fulfilled those criteria. The comparability domain examined the baseline data of exposure and control group, which expect to have no significant difference. The research by Makraris et al. was rated 2 points due to matching the control and exposure group. Of a population of 1475 in this study, only 828 were included in the analysis because only 828 patients have been compared between the hysteroscopy and non-hysteroscopy groups. In contrast, the rest of the studies showed comparability of the cohort in their characteristic table. All included studies had a good outcome domain. The assessment of the outcome and length of follow-up of the study was described clearly in the method. The adequacy of the follow-up cohort from all studies was fulfilled due to no reporting attrition in the study of more than 10% of the participants.
Overall, the eight studies included 4679 patients with RIF, 1869 patients underwent hysteroscopy before starting IVF, and 2163 patients were allocated in the control group (patients without hysteroscopic evaluation before starting ovarian stimulation for IVF treatment). In this population, the average age of patients ranged from 25.39 to 38 years, with an average number of previous failed IVF cycles ranging from 2.4 to 4.04. The broad definition of RIF in each study protocol were patients who underwent two or more failed IVF cycles with a good-quality embryo, and clinical pregnancy was determined by using an ultrasound examination with hearth beating. During hysteroscopic examination, generally a rigid hysteroscope is generally used with a sheath diameter of 4 to 5 mm and a fore oblique lens of 22-30 degrees.
Data synthesis
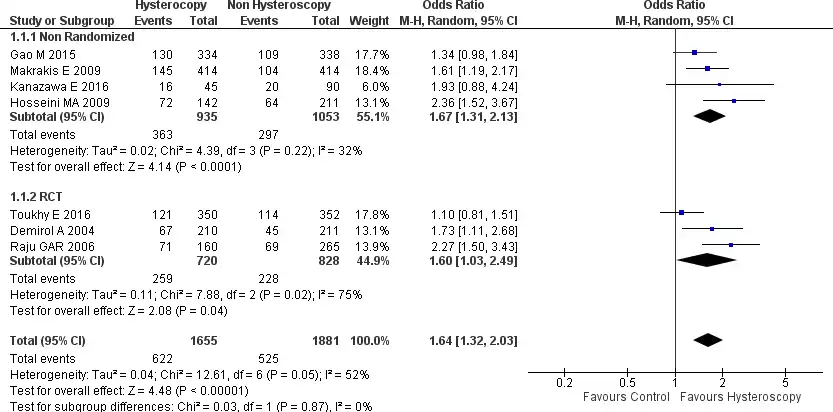
Seven of the eight studies, included in this meta-analysis, reported clinical pregnancy rate data (25-31). In the forest plot, the results of this analysis have a considerable heterogeneity between the seven studies included (Chi2=12.61, I2=52%), overall, this pooled analysis shows that the clinical pregnancy rate is significantly higher in patients who underwent hysteroscopy (HSC) when compared to the control group, which in this case is composed of patients with RIF who got IVF treatment without prior HSC examination [OR 1.64, 95% CI (1.32-2.03) p<0.001, (figure 3). Here we can also see the included results of the subgroup analysis of the non-randomized trial studies. The subgroup analysis shows that patients undergoing HSC have a higher clinical pregnancy rate [OR 1.67, 95% CI (1.31-2.13) p<0.0001]. The same was seen in the results of the RCT research analysis here also the clinical pregnancy rate was significantly higher in the HSC group [OR 1.60, 95% CI (1.03-2.49) p=0.04]. Both these results are presented in Figure 3.
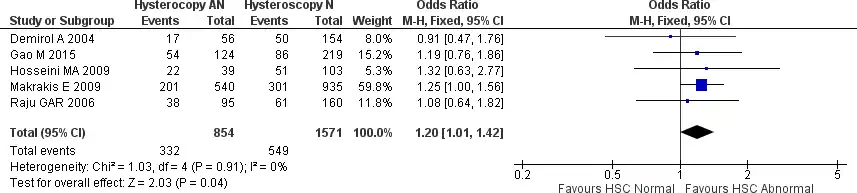
We also did analyze further a subgroup between patients with normal and abnormal hysteroscopy findings to see if there is any difference in clinical pregnancy outcome. Five studies were included in the analysis, with no heterogeneity noted (Chi2 = 1.03, I2 = 0%). This analysis did find that patients with abnormal hysteroscopy findings and treated accordingly have a marginally significantly higher clinical pregnancy rate [OR 1.20, 95% CI (1.01-1.42) p=0.04]. The forest plot is presented in Figure 4.
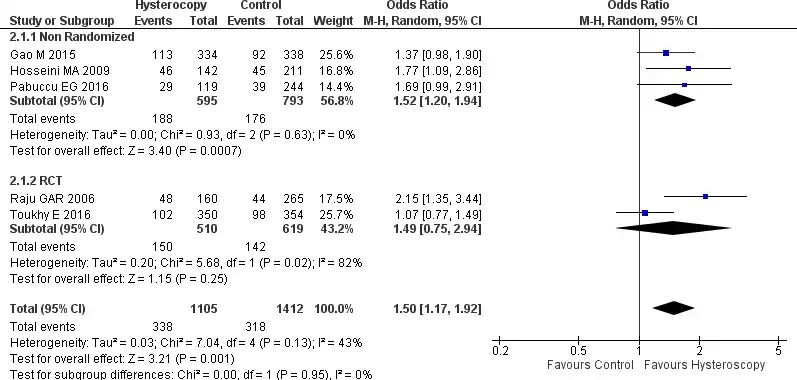
Five studies provided data regarding the live birth rate; three were non-randomized trials, and two were RCTs (27-29,31,32). Overall, a moderate heterogeneity was found between the five studies (Tau2 = 0.03, Chi2 = 7.04, I2=43%). The pooled forest plot analysis showed that patients with RIF who underwent HSC before starting IVF have a higher live birth rate [OR 1.50, 95% CI (1.17-1.92) p=0.001]. Subgroup analysis of the non-randomized trials showed the same result, patients in the HSC group have higher live birth rates [OR 1.52, 95% CI (1.20-1.94) p=0.0007). But for the RCTs, the result showed no significant difference with OR 1.49, 95% CI (0.75-2.94), p=0.25. All the results are presented in Figure 5.
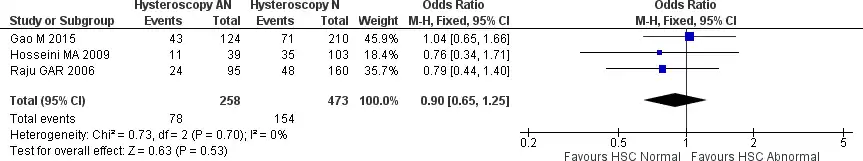
We further subgroup analysis between patients with abnormal and normal hysteroscopy findings to see whether there is any live birth rate difference. The analysis included three studies with no heterogeneity (Chi2 = 0.73, I2 = 0%). The forest plot found no significant difference between normal and treated abnormal hysteroscopy findings for live birth rate [OR 0.90, 95% CI (0.65-1.25) p = 0.53). The results are presented in Figure 6.
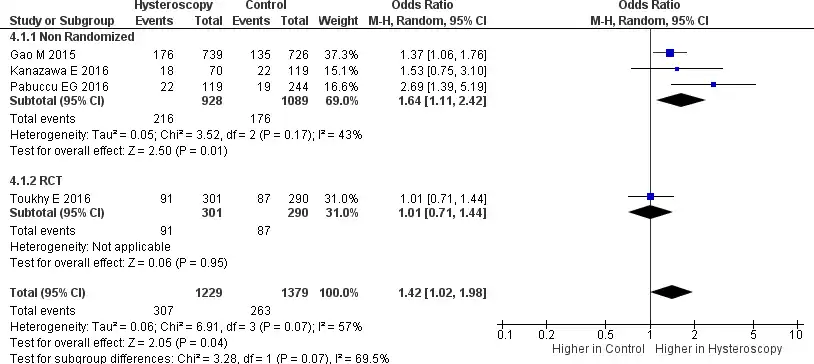
A total of four studies reported data related to the implantation rate, where 3 were non- randomized trials, and 1 was an RCT (28-31). This analysis shows considerable heterogeneity (Tau2 = 0.06, Chi2 = 6.91, I2 = 57%). In the forest plot provided in Figure 7, it can be seen that RIF patients who underwent HSC before IVF had a higher implantation rate [OR 1.42, 95% CI (1.02-1.98) p = 0.04]. The definition of implantation in this study is the number of gestational sacs divided by the number of embryos transferred. A subgroup analysis from a non-randomized trial also showed a higher implantation rate in patients undergoing HSC [OR 1.64, 95% CI (1.11-2.42) p=0.0005). Unfortunately, no RCT subgroup analysis can be done. because only one study provided data regarding implantation rate.
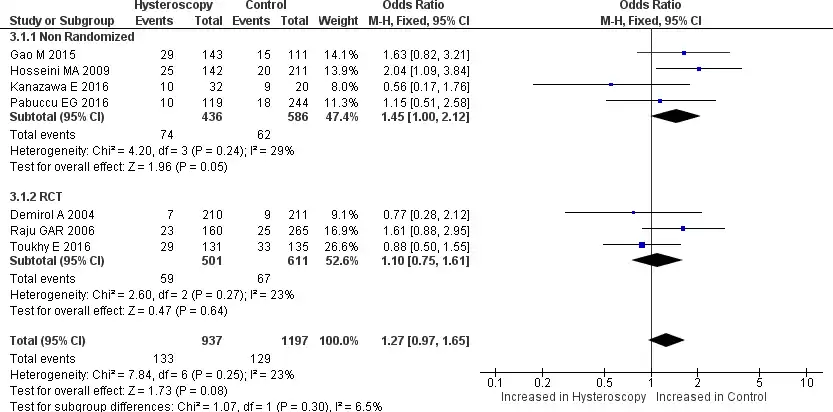
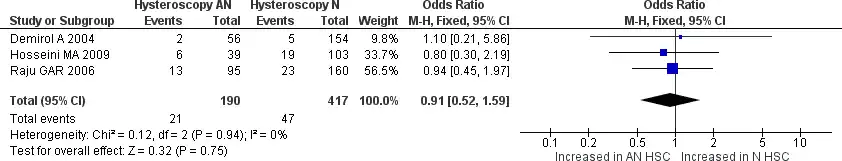
Seven studies reported miscarriage rate data; four were non-randomized, and the other three were RCTs. From the forest plot, the analysis had no significant heterogeneity among the studies (Chi2 = 7.84, I2=23%) (25,27,32). Pooled analysis showed that patients with RIF who underwent HSC before starting IVF had no significant miscarriage rate compared to patients with RIF who did not undergo HSC [OR 1.27, 95% CI (0.97-1.65) p=0.08]. Still, when we see the forest plot, the miscarriage rate does shift towards the control side, indicating that the miscarriage rate may be higher in the control group, but it is not statistically significant. The subgroup analysis of the non-randomized trial and RCT group also showed no significant difference [OR 1.45, 95% CI (1.00-2.12) p=0.05] and [OR 1.10, 95% CI (0.75- 1.61) p=0.64] respectively. All the results are provided in Figure 8. We also did further analyze the subgroup between normal and abnormal hysteroscopy findings. Three studies provided data regarding the difference between normal and abnormal hysteroscopy findings for miscarriage rate. No significant heterogeneity was found between the three studies. Pooled analysis shows no significant difference between normal and abnormal hysteroscopy findings for miscarriage rate [OR 0.91, 95% CI (0.52-1.59) p=0.75]. The results are provided in Figure 9.
Discussion
There are two main findings of this meta-analysis study. First, patients with RIF who underwent HSC prior to the IVF procedure were associated with improved clinical pregnancy -, live birth -, and implantation rate. Second, the subgroup analysis of patients with normal vs. abnormal HSC findings suggests that HSC had a significantly greater effect on the clinical pregnancy rate for patients with abnormal HSC findings. The broadly used RIF definition of the studies included patients who had two or more failed IVF cycles with good-quality embryos, with an average number of previous failed IVF cycles ranging from 2.4 to 4.04. Overall, our results demonstrate that HSC has a role in improving pregnancy outcomes in patients with RIF.
IVF has widely known as the most common ART procedure performed worldwide (33). In the late 70s, the first successful IVF treatment in humans was performed in England, with a laparoscopic retrieval of a single oocyte from the ovary. The oocyte was fertilized in vitro and transferred into her uterus as an embryo (34). Since then, IVF technology has advanced and become more widely available. In most cases, ART is used to treat infertility. Infertility is frequently correlated with anatomical and physiological abnormalities of the ovaries, fallopian tubes, and uterus. Based on the intrauterine pathologies, IVF can be performed by bypassing the affected area. For example, IVF bypasses the fallopian tubes directly in patients with tubal factor infertility (33-36). Thus, evaluating the intrauterine pathologies for IVF success is crucial.
Repeated or recurrent implantation failure (RIF) is a problem that has baffled many experts for quite a long time in the IVF environment and has been attributed to embryo quality and decreased endometrial receptivity. One of the suspected causes of RIF is specific issues in the uterine cavity, such as the inadequacy of endometrial thickness, adhesions, and anatomical abnormalities. Endometrial and uterine pathologies such as endometrial hyperplasia, polyps, leiomyoma, and endometriosis have been reported to occur in 18%-50% of women with RIF (17,36,37). Because of this, it is recommended to examine intrauterine pathologies before starting IVF. Several options that are often performed and are not invasive are a combination of transvaginal sonography, hysterosalpingography and hysteroscopy. But unfortunately, hysterosalpingography has low specificity, high false-negative, and high false-positive rates. Although transvaginal sonography is a noninvasive option, the results are less sensitive (6,10,38,39).
A more effective method for simultaneously evaluating the uterine cavity and providing treatment is hysteroscopy (40). As a result, HSC is the gold standard for evaluating the uterine cavity (13,14). In women with unsuccessful IVF treatments, HSC examination of the uterus is beneficial. A recent study reported that in patients whose transvaginal sonography examination results were normal, it turned out that during HSC examination, there were minor intrauterine abnormalities as high as 30%- 45%, and abnormalities found during HSC were significantly higher in patients who had a history of ART failure (15,17). This explains why many specialists perform HSC as the initial routine exam on patients with infertility despite the guideline recommendation (41).
A continuous process, starting with a successful implantation, establishing a clinical pregnancy, and ending with the delivery of a live baby, demonstrates the success of IVF. In our analysis, RIF patients that previously underwent HSC examination had higher clinical pregnancy rates [OR 1.64, 95% CI (1.32-2.03) p<0.001]. Subgroup analysis also did reveal that patients with abnormal hysteroscopy and had been treated for the latter did have higher pregnancy rate and a higher live birth rate [OR 1.50, 95% CI (1.17-1.92) p=0.001] but no significant difference could be demonstrated between patients with abnormal hysteroscopy findings compared patients with normal hysteroscopic findings.
The RIF patients who did undergo HSC before IVF also had a higher implantation rate [OR 1.42 (95% CI 1.02-1.98, p = 0.04]. These results align with a study conducted by Gao M, where it was found that RIF patients who underwent HSC had a significantly higher implantation rate (28). Uniquely, the study did not find a significant difference in the implantation rate in patients between abnormal and abnormal HSC findings. This can be caused because HSC can see minor lesions such as endometrial dysfunction and hyperplasi, polyps and adhesions that may occur due to ovarian stimulation and repeated intrauterine operations; this procedure can cause minor tissue damage (26). Besides that, HSC is said to be able to favor subsequent pregnancy outcomes. During HSC, cervical dilatation occurs, which allows the correction of cervical stenosis and facilitates the ET process, and uterine distention fluid can help flush the uterine cavity. The absence of a significant increase in the implantation rate in patients with abnormal HSC findings could be due to immune factors or poor embryo development (42).
The conclusion is that hysteroscopy has a fertility-enhancing effect, which is also thought to occur independently of the correction of intrauterine abnormalities. Hysteroscopy is also believed to improve ART outcomes through an endometrial injury that helps embryo implantation (42).
Strengths and Limitations; The strengths of our analysis were that we did include RCT studies with a high level of evidence; we also included two-arm cohorts and retrospective studies, of an overall good quality. Our results were generally consistent across the studies when we saw the primary endpoints, which ensured consistency in each study. This meta-analysis has several limitations, apart from the relatively small number of studies that could be included. Important is to note that patient demographics and procedure differences, that should have been accounted for in this analysis, may influence the outcome and which may also increase heterogeneity. It should also be remembered that some of the results of this study have considerable heterogeneity; this is quite difficult to correct in a meta-analysis study because we cannot control every population in each study. Therefore, there is a possibility of bias that we cannot control.
Conclusion
Overall, this meta-analysis shows that hysteroscopic examination in patients with RIF before IVF significantly improved clinical pregnancy -, implantation-, and live birth rates. Also, to our knowledge, this meta-analysis is the first to look at differences in patients with abnormal and normal hysteroscopic findings, but unfortunately, no significant differences could be brought to evidence. Although many studies have been related to the role of hysteroscopy in patients with RIF before starting IVF treatment, additional studies are still needed, especially large-scale RCT studies.
References
Figure 2: preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram.
Figure 3. Forest plot of clinical pregnancy rate. Odd ratio of clinical pregnancy rate between patients with RIF who underwent hysteroscopy before IVF and did not undergo hysteroscopy before IVF. Test for overall effect: Z = 4.48 (p<0.0001) heterogeneity: I2 = 52%. CI, confidence interval; RCT, randomized clinical trial.
Figure 4. Forest plot of clinical pregnancy rate between normal and abnormal hysteroscopy findings in patients with RIF who underwent hysteroscopy before IVF. Test for overall effect: Z = 2.03 p=0.04 heterogeneity: I2 = 0%. HSC: hysteroscopy.
Figure 5. Forest plot of live birth rate. Odd ratio of live birth rate between patients with RIF who underwent hysteroscopy prior to IVF and the patients who did not undergo hysteroscopy prior to IVF. Test for overall effect: Z = 3.21 (p=0.001) heterogeneity: I2 = 43%. CI, confidence interval; RCT, randomized clinical trial.
Figure 6. Forest plot of live birth rate between normal and abnormal hysteroscopy findings in patients with RIF who underwent hysteroscopy prior to IVF. Test for overall effect: Z = 0.63 p=0.53 heterogeneity: I2 = 0%. HSC: hysteroscopy.
Figure 7. Forest plot of implantation rate. Odd ratio of implantation rate between patients with RIF who underwent hysteroscopy prior to IVF and the patients who did not undergo hysteroscopy prior to IVF. Test for overall effect: Z = 2.05 (p=0.04) heterogeneity: I2 = 57%. CI, confidence interval; RCT, randomized clinical trial.
Figure 8. Forest plot of miscarriage rate. Odd ratio of miscarriage rate between patients with RIF who underwent hysteroscopy prior to IVF and the patients who did not undergo hysteroscopy prior to IVF. Test for overall effect: Z = 1.73 (p=0.08) heterogeneity: I2 = 23%. CI, confidence interval; RCT, randomized clinical trial.
Figure 9. Forest plot of miscarriage rate between normal and abnormal hysteroscopy findings in patients with RIF who underwent hysteroscopy prior to IVF. Test for overall effect: Z = 0.32 p=0.75 heterogeneity: I2 = 0%. HSC: hysteroscopy.
Table 1. Modified Newcastle-Ottawa Scale for Cohort Study
Figure 1. Quality assessment of RCT. A: risk of potential bias of individual RCT studies. B: risk of bias summary of all RCT studies. RCT: randomized wontrolled trial.
Table 2. Base Summary of Study Characteristics
Table 3. The Base Summary of Hysteroscopy Examination Procedure, Ovarian Stimulation Procedure, and Embryo Transfer Procedure