Authors / metadata
DOI : 10.36205/trocar3.2023001
Article
Historically, the commonly used treatment for gynecological benign anatomical entities has been excisional, that is, surgical resection to remove the lesion. In the past 30 years, excisional treatment has gradually become minimally invasive. Laparoscopic and hysteroscopic surgery have become the treatments of choice for many gynecological diseases. It is also known as the era of minimally invasive gynecological surgery (1- 4). Minimally invasive surgery, compared with traditional open surgery, is less painful, recovery is faster, produces smaller scars, and yields a higher patient satisfaction (5-7).
The improvement of the technology always promotes the development of lesser invasive treatment solutions, here the appearance of ablation therapy provides a less invasive option for uterine benign diseases such as fibroids and adenomyosis.
Ablation therapy, also called myolysis in case of myoma, is not a specific technology, it is a general denomination for a group of technologies, the basic principle of which is to use high temperature or low temperature to destroy the lesion (8-11). At this moment in time, thermal ablation is more commonly used, and cryoablation is also used in some tumors such as lung tumors (12-13).
The energy source for generating heat can be high-intensity focused ultrasound, microwave, radio frequency, or others. After the energy is generated, whether tissue degeneration and necrosis occur is related to the temperature of the target tissue after the energy is applied (14). If the local temperature in the tissue reaches 65 °C, it can achieve tissue damage within one second, leading to tissue degeneration and necrosis, and if the temperature reaches 54 °C, it takes 3 seconds to achieve tissue damage (15-16).
High intensity focused ultrasound (HIFU) is a method of tissue damage using ultrasonic energy, similar to the principle of a solar power cooker. A solar power cooker focuses sunlight on the cooker, while HIFU concentrates the energy of ultrasonic waves on the focal point in the tissues. This is a way to treat the tumor without touching it. Over the treatment path, because of the fact that the accumulation of energy does not reach a sufficient temperature needed to destroy tissues, it will therefore not cause damage to these tissues, and because the energy at the focal point is high, when it reaches above 65 °C, the tissue will be destroyed instantly (17-18).
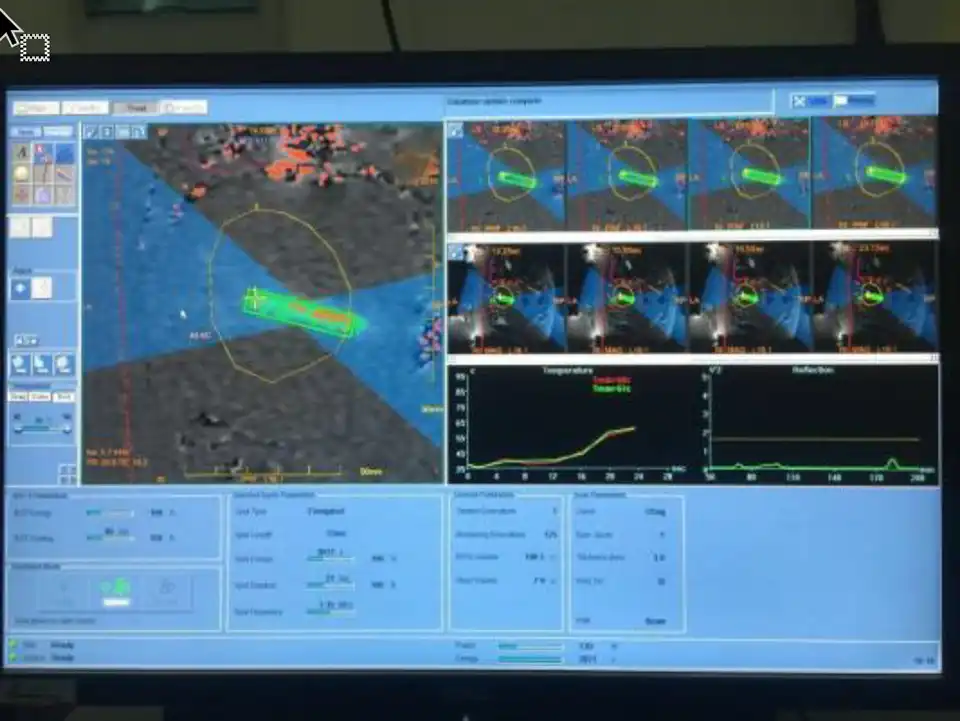
At present, HIFU includes ultrasound- guided treatment (e.g., Haifu® Chongqing Medical Technology Co Ltd Chongqing China) and Magnetic Resonance Imaging (MRI)-guided Focused Ultrasound (MRgFUS) treatment (e.g., Exablate® Insightec STel Aviv – Yafo, Israel) (19). The two technologies have their own advantages and disadvantages. Ultrasound-guided high intensity focused ultrasound therapy is relatively convenient for adjusting the body position and designing treatment paths because of the real-time nature of the ultrasound signals. For MRgFUS, the image quality is better than the ultrasound-guided, there is also a temperature monitoring system during the treatment process, that can control the treatment boundary more accurately and ensure safety, but the operation time will be relatively longer (20) (Fig 1). The energy used by HIFU is ultrasound for the treatment of uterine diseases, fibroids and adenomyosis, Ultrasound can reach the uterus through the skin, the subcutaneous tissue, and the peritoneum. If the uterus is in the anterior position and very close to the abdominal wall, the treatment is easy to perform. If there are bowel loops in front of the uterus, then HIFU cannot be performed because ultrasound cannot pass through the air inside the bowel loops. Sometimes the intestines can be pushed aside by a full bladder, and ultrasound can travel through the bladder to perform the treatment. Since HIFU treatment works in a ” telekinetic” fashion, its advantages include no scars, minimal trauma, quick recovery, and almost no bleeding during the treatment process.
The treatment can be carried out in outpatient clinics, but pathological examinations are not available during the treatment process, nor can it be combined with laparoscopic or hysteroscopic techniques. In addition, in order to avoid nerve injure during the treatment process, general anesthesia or regional block anesthesia cannot be used. Intraoperative sedation and analgesia are the only options, and most patients may experience a certain discomfort during the treatment process.
Microwave ablation is practiced by using a 16-18G sized needle like antenna to generate high-temperature energy within the punctured target tissue, causing destruction to the target tissue. There is water circulation within the microwave antenna sheath that does reduce the temperature of the rod. Therefore, on the puncture path the tissue temperature will not reach the destructive effect, this system can avoid damage to normal tissue.
Microwave ablation for the treatment of uterine-related diseases is usually performed under the guidance of abdominal ultrasound. The abdominal ultrasound probe guides the microwave antenna to puncture the uterus through the skin. Like HIFU, when the anterior positioned uterus is close to the abdominal wall, it can be treated by percutaneous microwave ablation. Different from HIFU, during percutaneous microwave ablation, the bladder, with the patient in lithotomy position, can be used and a uterine manipulator can be placed to change the position of the uterus during the treatment, in doing so a safe treatment pathway can be achieved. In some patients, when the uterus is in retroversion, or the lesion is relatively close to the posterior wall, an abdominal ultrasound-guided transcervical puncture or a transvaginal ultrasound guided transcervical puncture can be performed to reach the target area for treatment.
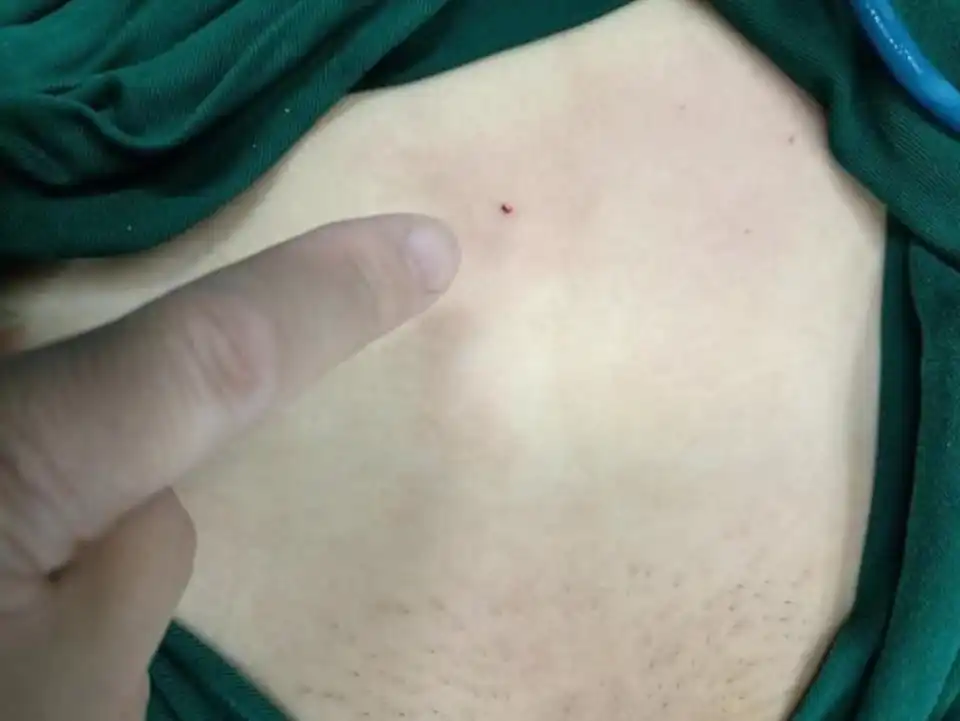
Laparoscopic microwave ablation can be performed on posterior adenomyotic lesions. Here a greater degree of destruction of the adenomyotic lesions can be achieved. Another difference from HIFU is that the microwave ablation is usually performed in the operating room, and the anesthesia can be general anesthesia or regional block anesthesia. The patient will not feel any pain during treatment. Another advantage is that a needle biopsy can be performed simultaneously to obtain pathology. Compared with HIFU, percutaneous microwave ablation has a puncture point, but the puncture needle hole is very tiny, and basically there is not much bleeding during the treatment process. Compared with laparoscopic surgery, there is no pneumoperitoneum effect, and the trauma is therefore much more reduced (Fig 2).
Another type of ablation energy is radiofrequency. Its working principle is to use high-frequency current to generate molecular oscillations to destroy target tissue. The principle and the method of percutaneous puncture are similar to percutaneous microwave ablation. At present, there are companies in the Chinese market that design transcervical radiofrequency ablation probes that can penetrate through the cervix to the target tissue. An advantage is that the probe is reusable. Compared with microwave, the energy of radio- frequency is slightly lower, and it works slower on larger lesions with a diameter above five cm. One advantage of radio frequency is that it can automatically terminate the ablation through impedance detection. Once the resistance of the target tissue reaches a certain threshold; it will stop working.
Ablation therapy is guided by ultrasound or MRI. Generally speaking, lesions with diameters smaller than two cm usually cannot be accurately located and cannot be ablated, while lesions with diameters larger than ten cm can increase the difficulty of the treatment, especially for HIFU therapy. It will take too long a time and often requires GnRH-a pretreatment.
Before treatment, in addition to evaluating the size and location of uterine fibroids and adenomyotic lesions using ultrasound, it is necessary to check whether there are intestinal loops on the puncture path before implementing high intensity focused ultrasound. If there are intestinal loops that cannot be pushed aside, HIFU cannot be performed. Instead, when microwave or radiofrequency methods are used, if the abdominal path cannot be set up, a cervical or vaginal puncture route to avoid interference of the intestines can be tried.
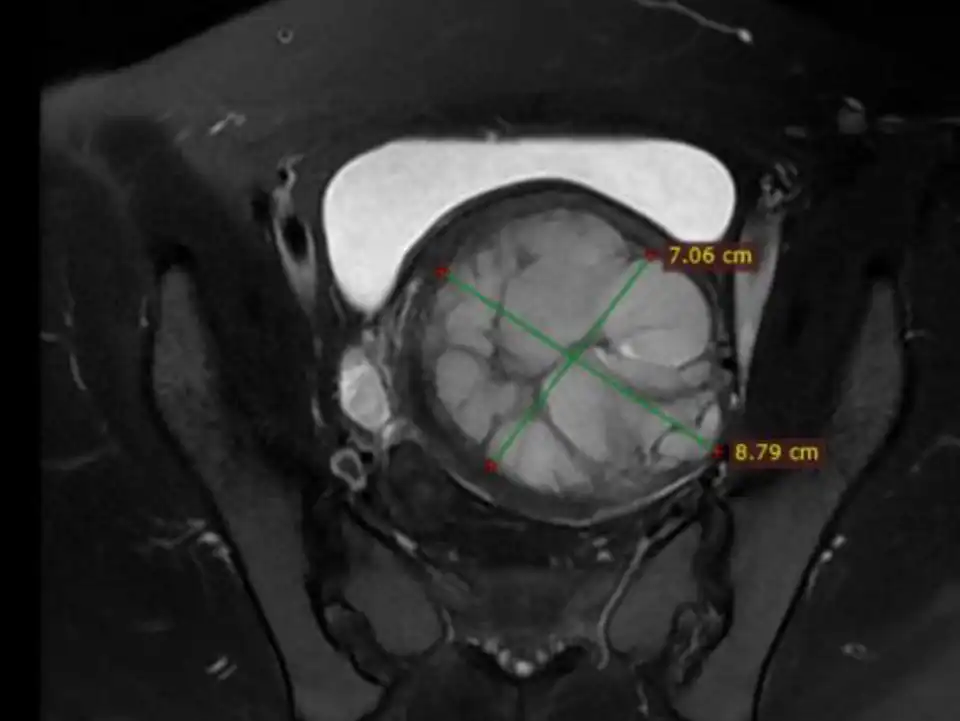
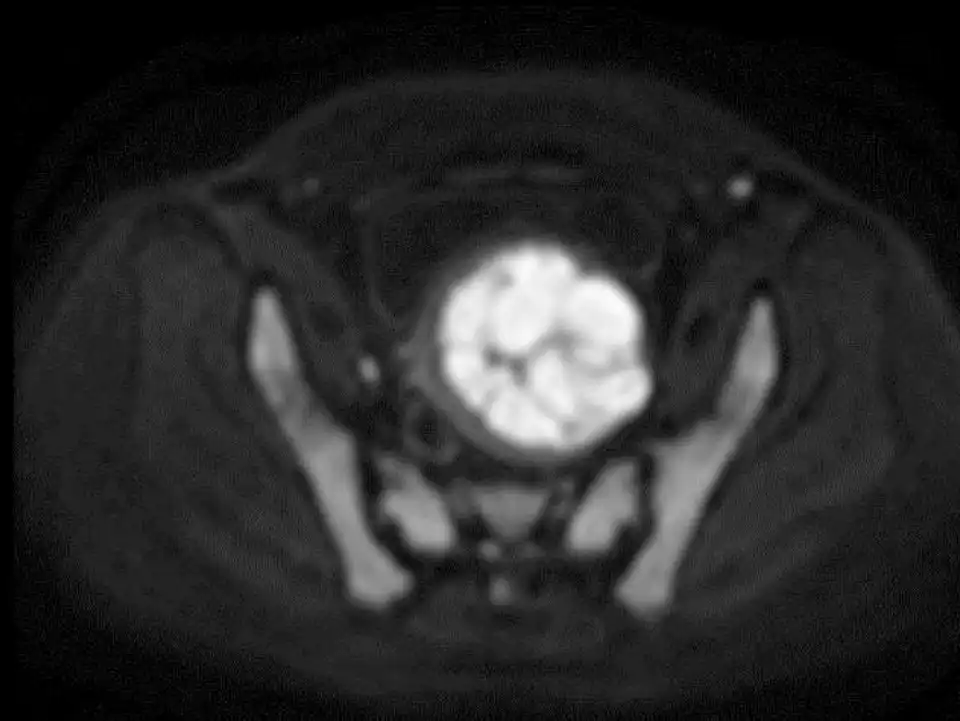
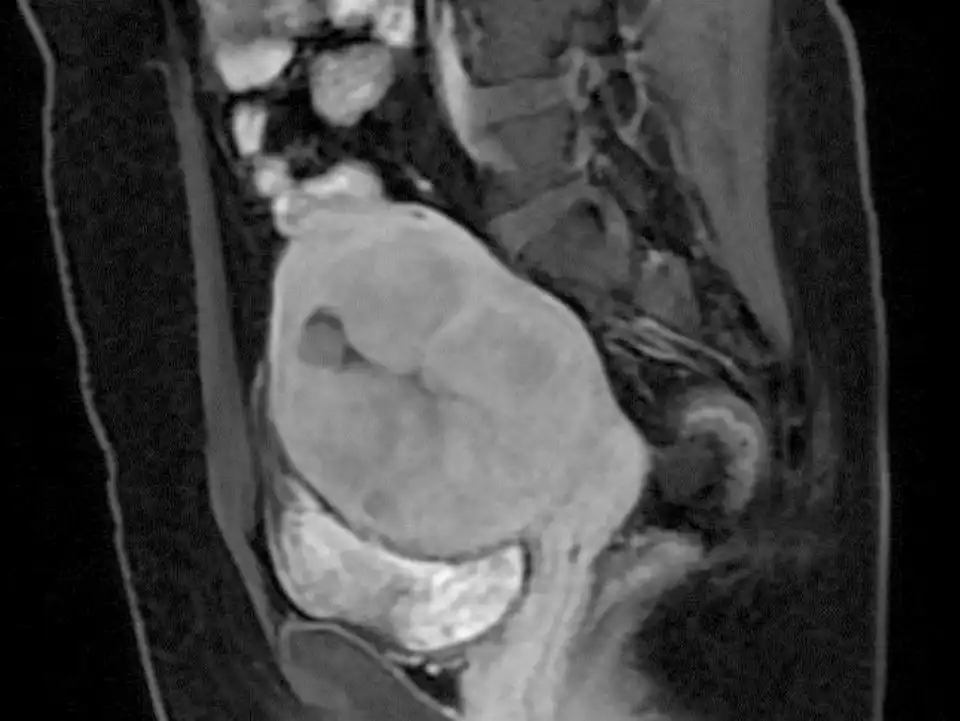
Preoperative plain scan and contrast enhanced MRI can differentiate uterine fibroids from undetected uterine leiomyosarcomas (Fig 3-6). High Diffusion Weighted Imaging (DWI) signal intensity, low Apparent Diffusion Coefficient (ADC) and uneven enhancement are the signals of uterine sarcomas (21). The T2 weighted i image (T2WI) signal of MRI can also evaluate the difficulty of ablation. If the T2WI signal is high, it indicates that the water content in the fibroid is high, and it is often more difficult to perform ablation than in those with T2WI low signal intensity (22).
For uterine fibroids, if microwave or radiofrequency ablation treatment is performed, a 14 G biopsy needle can be used to obtain tissue for pathological diagnosis before treatment. In contrast HIFU treatment often makes it inconvenient to perform simultaneously a biopsy for pathology due to the patient’s body position.
For patients with submucosal fibroids or other intrauterine lesions such as endometrial polyps, it is convenient to perform hysteroscopic examination and treatment simultaneously during microwave or radiofrequency ablation. Therefore, for patients with multiple uterine leiomyomas, we often prefer to use hysteroscopy to remove the type 0-2 lesions simultaneously, while for type 3-7 lesions, ablation can be used. For patients with menorrhagia, it is also convenient to perform endometrial ablation simultaneously to reduce menstrual flow and relieve symptoms. Likewise, hysteroscopic examination or treatment cannot be performed concurrently with HIFU due to patient’s position and lack of anesthesia.
Different is the ablation treatment of some malignant tumors. The ablation of malignant tumors requires the implementation of over-range ablation, that is, the area of ablation necrosis should not only include the tumor, but also include the tissue of about half to one cm around the tumor to ensure the effectiveness and thoroughness of the treatment. In parenchymal organs, such as lung, liver, and thyroid, because of the protection of surrounding tissues, over-range ablation is usually safe.
For ablation of benign uterine diseases, the first priority is to ensure the safety of the treatment. The organs surrounding the uterus, such as the intestines and bladder should not be injured by ablation, otherwise this will gender or cause a complication. For patients with fertility requirements, the ablation treatment also needs to ensure that the scope of ablation is limited to the inside of uterine fibroids or adenomyotic lesions to avoid damage to the endometrium or the uterine seromuscular layer, otherwise there may be uterine adhesions, infertility, or uterine rupture during pregnancy after treatment (23-24).
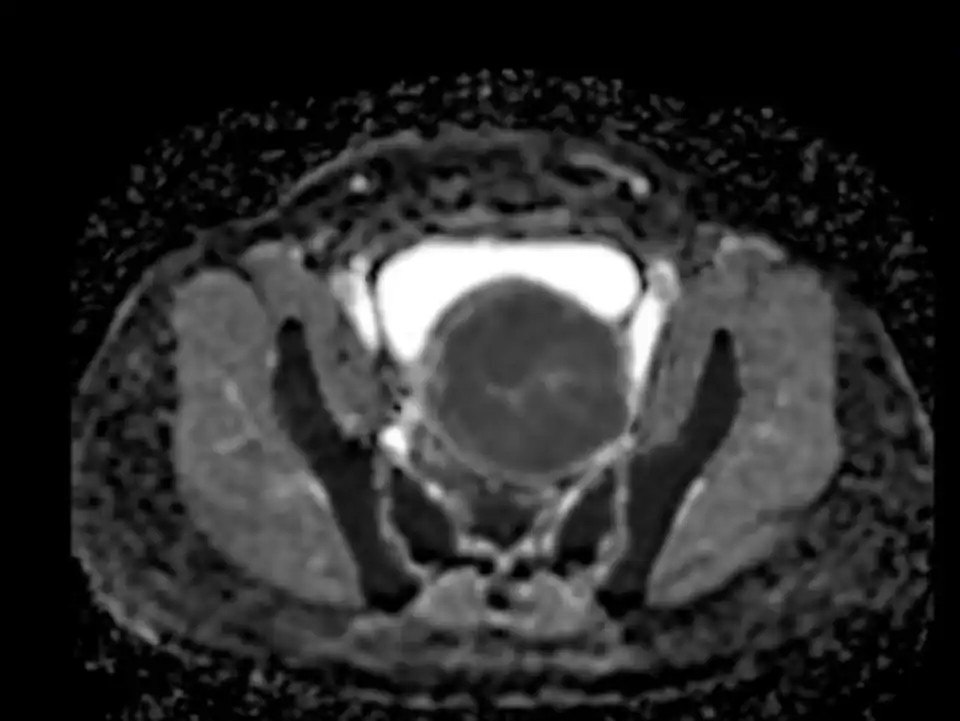
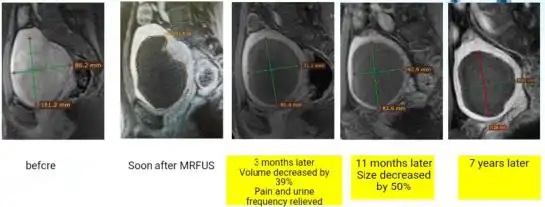
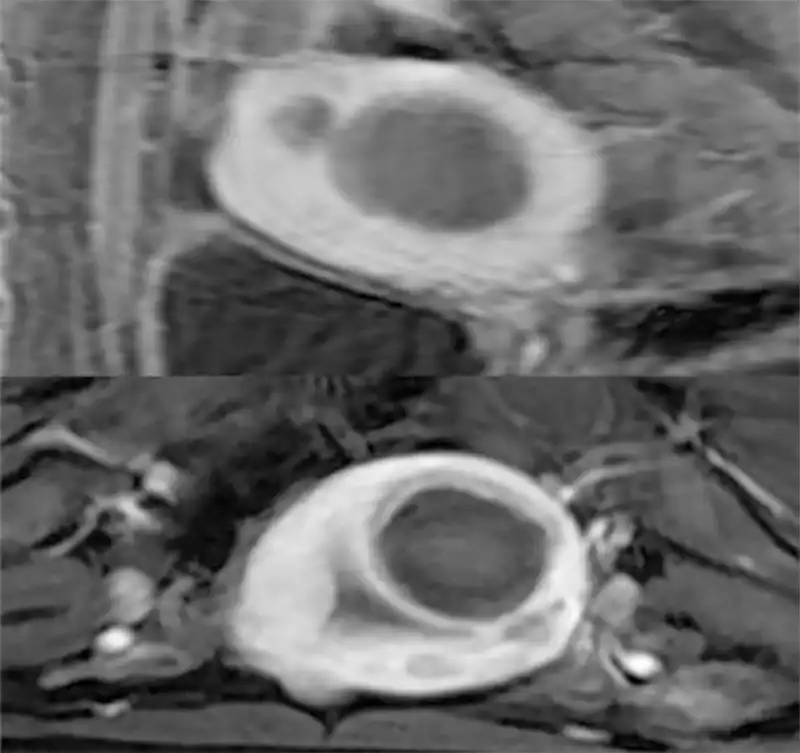
Different from excisional treatment, the postoperative lesion cannot be seen by ultrasound or MRI. After ablation, the lesion will gradually undergo necrosis and will be absorbed by the blood circulation, leading to atrophy of the lesions. If ultrasonic detection is used, it is often impossible to detect necrosis inside the lesion. By contrast enhanced ultrasound or contrast enhanced MRI, necrosis inside the lesions can be detected. Usually, we use non-perfused volume (NPV) to describe the proportion of necrosis after ablation (25) (Fig 7). The larger the NPV, the more symptoms will be cured. After ablation, usually the lesions gradually shrink, and symptoms are gradually cured (Figure 7). Uterine necrotic lesions often appear as degenerated fibroids on postoperative ultrasound.
Because the lesions do not completely disappear after ablation, we generally do not use the recurrence rate to evaluate the treatment efficacy. If it is considered that the presence of adenomyosis or uterine fibroids on ultrasound is a recurrence, then we can assume that the recurrence rate is almost 100%. With ablation therapy, we usually prefer to use the re-operation rate to evaluate the efficacy after treatment.
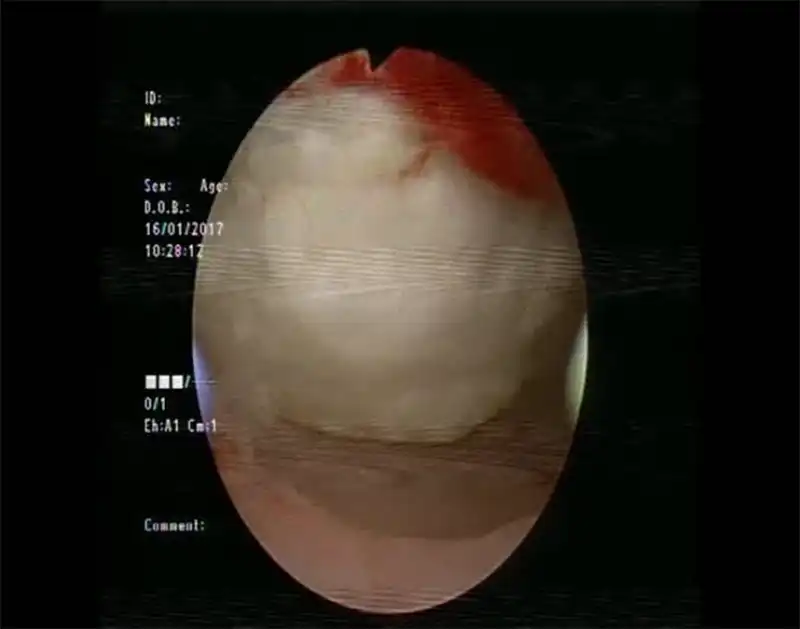
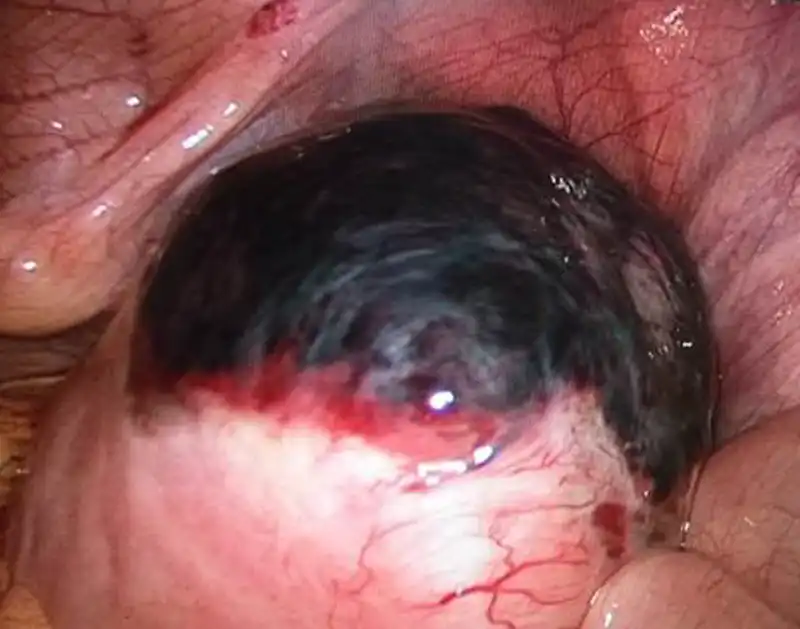
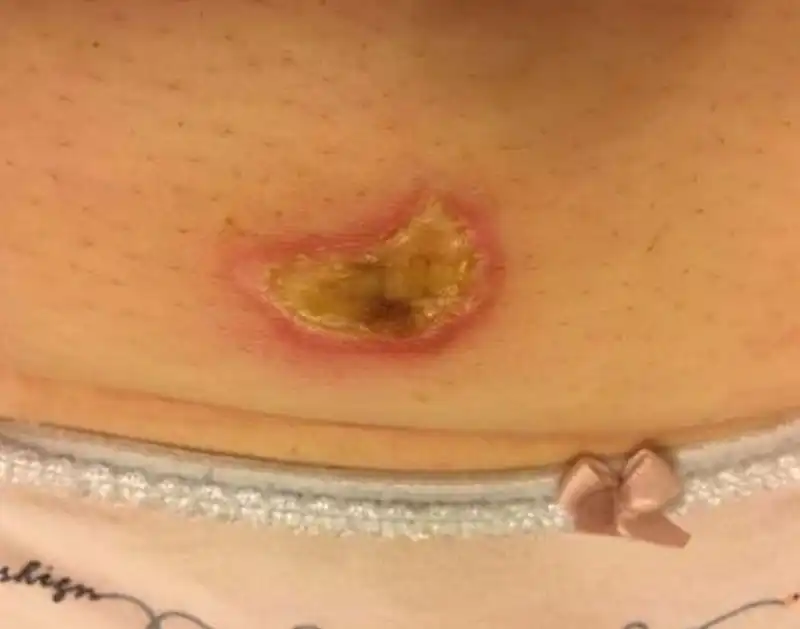
When it comes to the complications of ablation therapy, injury to adjacent organs caused by overtreatment is an important aspect. In addition to the injury to the endometrium (Fig 8) or the seromuscular layer (Fig 9) of patients wishing fertility, injury to other organs is also an important aspect,including bowel loops (1-3‰), bladder, skin and subcutaneous tissue. The injury to the bowel is often delayed, occurring 5-10 days after ablation. Heavy bleeding may occur after treatment due to vessel injury or uterine bleeding in some cases. Skin burn may happen during HIFU and microwave treatment (Fig 10). Nerve injury is a unique complication of HIFU therapy, which is related to the backfield energy of focused ultrasound behind the target tissue, which is also the reason why we use intravenous sedation and analgesia during high intensity focused ultrasound therapy. However, there are still a small number of patients with transient nerve involvement, manifested as sensory or motor disturbances in the legs.
Infection after ablation therapy is relatively higher than resection treatment, especially when ablation combined with some therapeutic procedures in the uterine cavity. Fever, abdominal pain, and odor discharge are the main symptom after infection. Antibiotics are necessary for the treatment. Dilation and curettage or hysterectomy are needed when antibiotics cannot control infection. Prolonged watery discharge is common when ablation affects the endometrium.
Whether ablation therapy can be performed for patients with fertility wish needs further research. Current effective research suggests that ablation therapy is safe, but there are also reports of uterine rupture during pregnancy after ablation therapy (24-26-29-30). For patients with fertility wish, it is key to control the range of ablation therapy and not to over-treat when performing ablation therapy. During the treatment process, it is necessary to ensure that the integrity of the endometrium and seromuscular layer is not damaged to the maximum possible (Fig 11). It is better to have an insufficient ablation than secondary damage. After implementation of the ablation therapy, it is generally considered, empirically, that the patient can try to conceive one to three months after the treatment. A study on pregnancy after MRgFUS found that the complications of pregnancy after MRgFUS were comparable to those in the general population (31). Of course, due to the rarity of pregnancy complications more data have to be generated.
Compared with traditional laparoscopic surgery or open surgery, ablation treatment causes less damage to the patient, less pain, almost no blood loss or very minimal bleeding, and shorter time to recovery and return to work. Most treatments can be performed in outpatient or at day surgery centers without a long hospitalization. Due to the fact that benign uterine diseases such as uterine fibroids and adenomyosis are age- related diseases, the lesions tend to shrink and symptoms are reduced with the decrease of estrogen after menopause.
Therefore, for these benign diseases of the uterus, in addition to the traditional resection therapy, ablation therapy undoubtedly provides a new direction for the treatment. In our opinion, ablation therapy provides us with an alternative treatment for benign uterine disease alongside endoscopic surgery. It can also be considered as the next generation of surgical technology, which deserves to be mastered by more gynecologists.
Of course, compared with resection therapy, ablation therapy also has some disadvantages. For example, the lesion will not disappear completely after treatment. The chance of secondary surgery is relatively high, and the chance of secondary infection after treatment is relatively high.
Nevertheless, ablation therapy still provides a very good direction for the conservative treatment of benign gynecological tumors such as uterine fibroids and adenomyosis.
Video
References
Figure 1: Temperature monitoring during MRgFUS
Figure 2. The tiny puncture needle hole in percutaneous microwave ablation
Figure 3: MRI revealed a tumor of about 9 cm
Figure 4: DWI revealed high signal intensity
Figure 5: Contrast-enhanced MRI revealed that there was necrosis inside the tumor.
Figure 6: MRI revealed that the ADC value of the tumor was low
Figure 7. Non-Perfused Volume (NPV) area after ablation
Figure 8. Endometrial damage by HIFU (figure courtesy of Dr. Ma Ning, Beijing Fuxin Hospital).
Figure 9 Laparoscopy View of uterine damage caused by HIFU
Figure 10. Skin injury cause by microwave ablation
Figure11. Enhanced MRI did show the ablation zone after MRgFUS