Authors / metadata
DOI: 10.36205/trocar1.2023013
Abstract
Isthmocele is an anatomo clinical entity still lacking standardization in its diagnostic and therapeutic approaches. It is a condition where building bridges between ultrasound and hysteroscopy is particularly fruitful. The surgical management of the symptomatic forms can be conducted through hysteroscopic, laparoscopic, vaginal or laparotomic routes. In this article is provided a review of the relevant data of the literature exclusively related to its diagnostic aspects. An overview of the main hysteroscopic and sonographic findings encountered in practice is also discussed and provide new insights on the condition’s pathogenesis. Although the niche has long been thought of as a static defect causing accumulation of menstrual blood, it is also likely to act as an active valve closing the internal ostium of the cervix and causing fluid dynamic changes as described by an Indian team. Additional observations during hysteroscopy allow collecting different anomalies which are likely to explain the usually encountered symptoms such as abnormal uterine bleeding, subfertility, chronic pelvic pain, dysmenorrhea and dyspareunia.
Introduction
The isthmus is the lower uterine segment (LUS) lined by a poorly hormone-responsive endometrial mucosa, whose anatomic lower limit is the histological internal os of the uterus (commonly termed internal os of the cervix) and whose anatomic upper limit is the anatomical internal os of the uterus, first described in hysteroscopy in 2020 (1).
Isthmocele etymologically means hernia of the isthmus and by extension, it is the defect resulting from an impaired healing of both the endometrium and myometrium at the site of the cesarean incision. It is also termed cesarean scar defect (CSD), niche, pouch or diverticulum and was first described in these terms in 1995 by Hugh Morris on hysterectomy samples of women who suffered of persistent abnormal uterine bleeding (AUB) without any other identifiable condition (2).
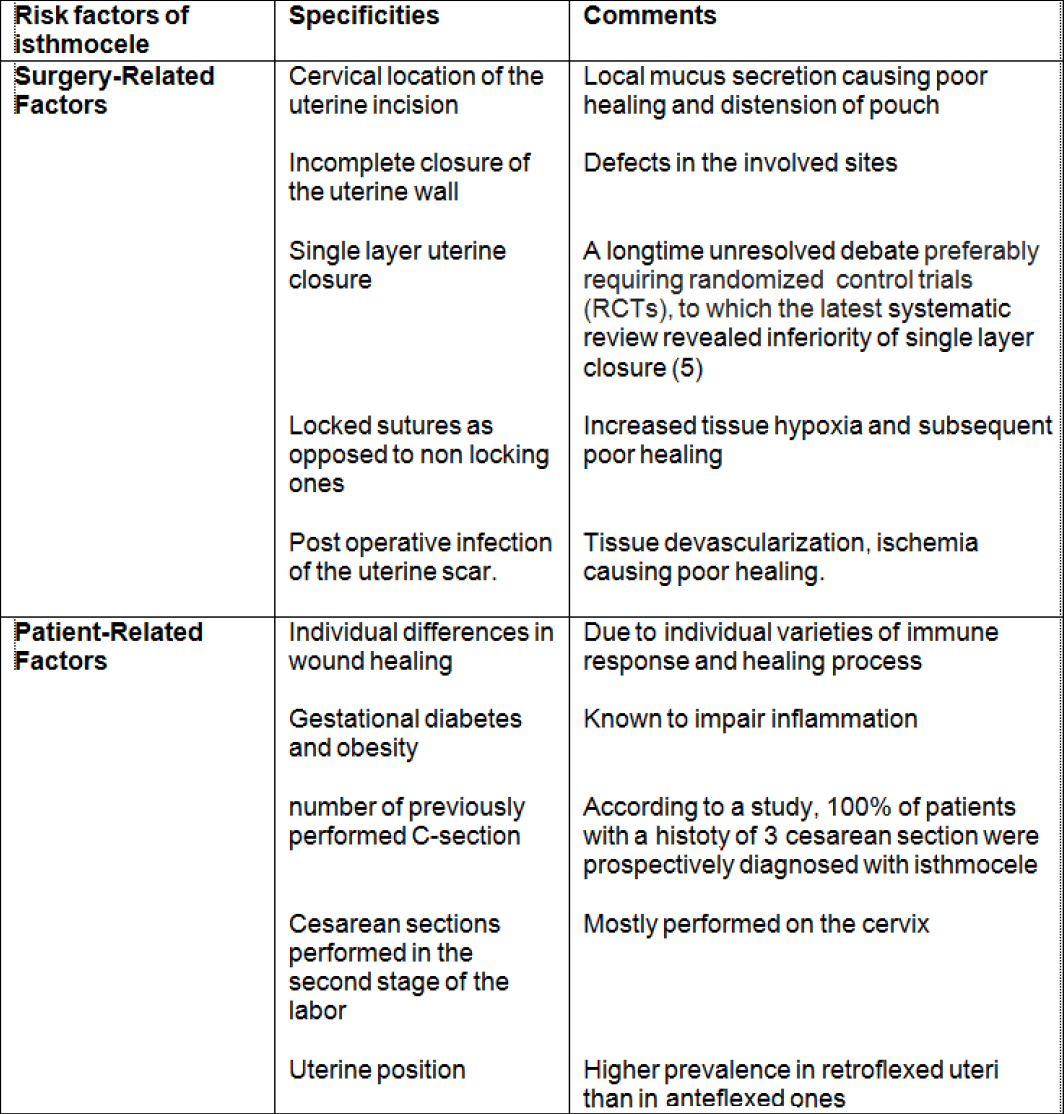
The prevalence of isthmocele ranges from 24% to 70% in transvaginal ultrasound (TVUS) examination according to some authors, although the exact number is unknown and difficult to assess (3). The risk factors so far associated with the genesis of a CSD are summarized in table 1 (4-6).
Many patients may be asymptomatic, yet in premenopausal women, post menstrual AUB is the most classic symptom with a variable impact on patients’ quality of life. Chronic pelvic pain, secondary subfertility, dysmenorrhea and dyspareunia are among the well-established consequences of this condition, let alone the potential obstetrical complications during an upcoming pregnancy such as scar pregnancy, placenta accreta and scar dehiscence (3,4,7).
In the persistent lack of high quality evidence and standardization of isthmocoeles diagnosis and treatment (8), this article aims to review the relevant data of the literature which are exclusively related to the condition’s diagnostic and etiopathogenic aspects, with a special focus on the hysteroscopic and sonographic findings encountered in practice and which provide additional insights on the pathogenesis of the commonly encountered symptoms, namely AUB, subfertility, chronic pelvic pain, dysmenorrhea and dyspareunia.
Simple isthmocele: a triangular defect collecting menstrual blood
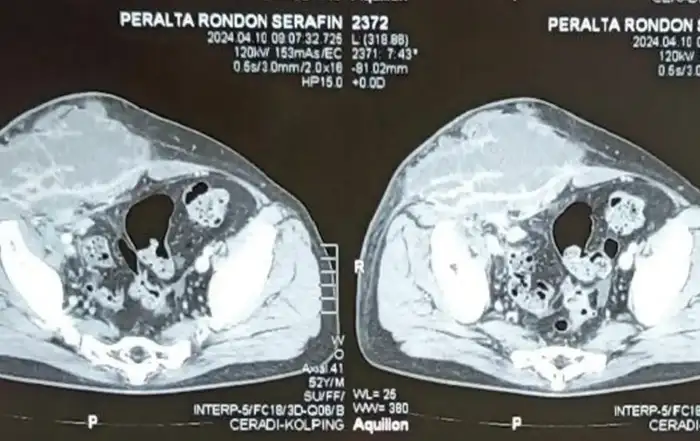
Posing the diagnosis of isthmocele starts with a two-dimensional (2D) ultrasound for the visualization of the defect, its length from up to down, its 2 mm minimum depth as well as the residual myometrium (9). It is located inside the cervix, the isthmus or the cervico-isthmic level. Three-dimensional (3D) ultrasound additionally provides the lateral width of the pouch and in case of difficulties; saline/gel infusion sonography allows clearer visualization through distension.
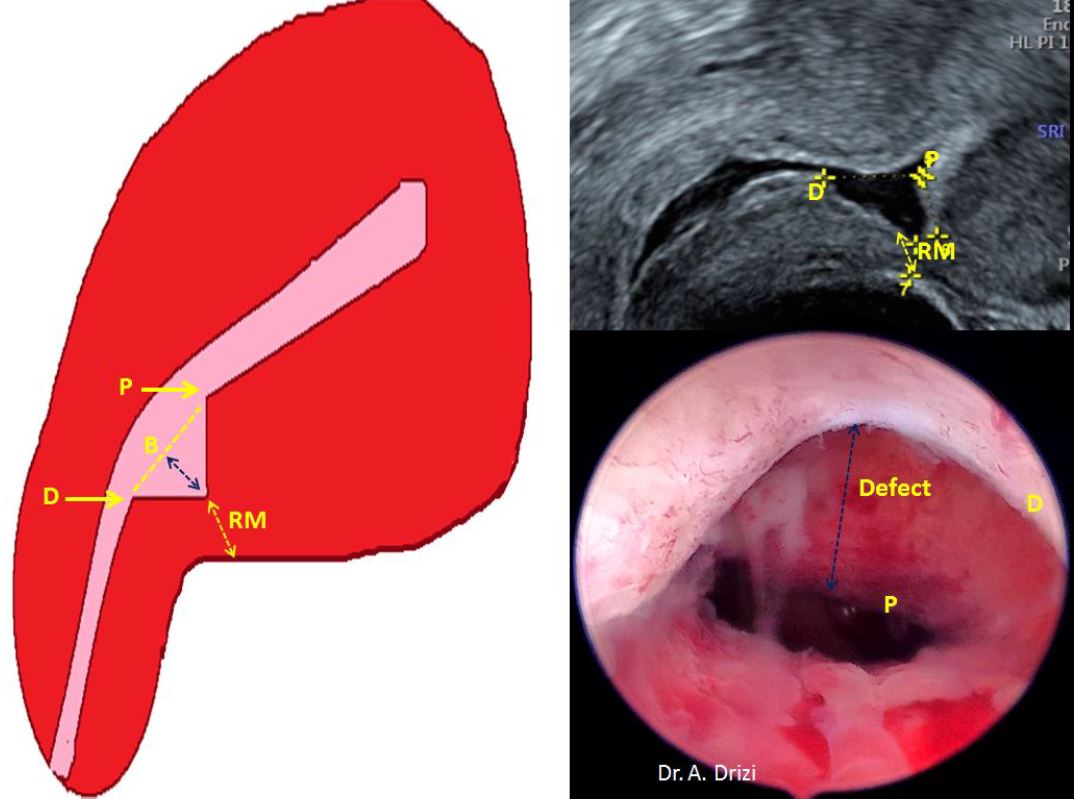
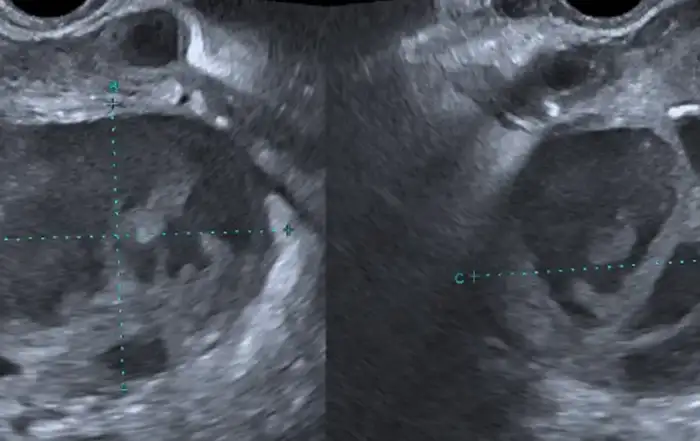
At the thought of the term isthmocele, the ultrasonographic image of a triangular anechoic area in the LUS comes to mind, as well as the thought of a pouch where menstrual bleeding is cyclically collected –due to reduced uterine contractility by to the presence of scar tissue– and subsequently released in the form of blackish or brownish spotting. This triangular shape is limited by a distal edge oriented towards the cervix; a proximal edge closer to the uterine corpus and a roof, on top of which lies the residual myometrium separating the defect from the serosa (10) (Fig 1).
The distention of the CSD by blood could be such that it mimics the uterine cavity when hysteroscopy is performed by a non-warned practitioner. This highlights the importance of proper irrigation and evacuation of blood during hysteroscopy, with a gentle progression of the scope and particular attention to the anatomical landmarks defining the uterine cavity, mainly the tubal ostia.
The persistence of menstrual blood in the cervico-isthmic level is described to negatively influence mucus quality as well as the environment for sperm transport and embryo implantation, also acting as a physical barrier in embryo transfer during assisted reproduction technologies (ART), secondarily causing infertility (8). However, there is lack of high-quality data regarding these mechanisms.
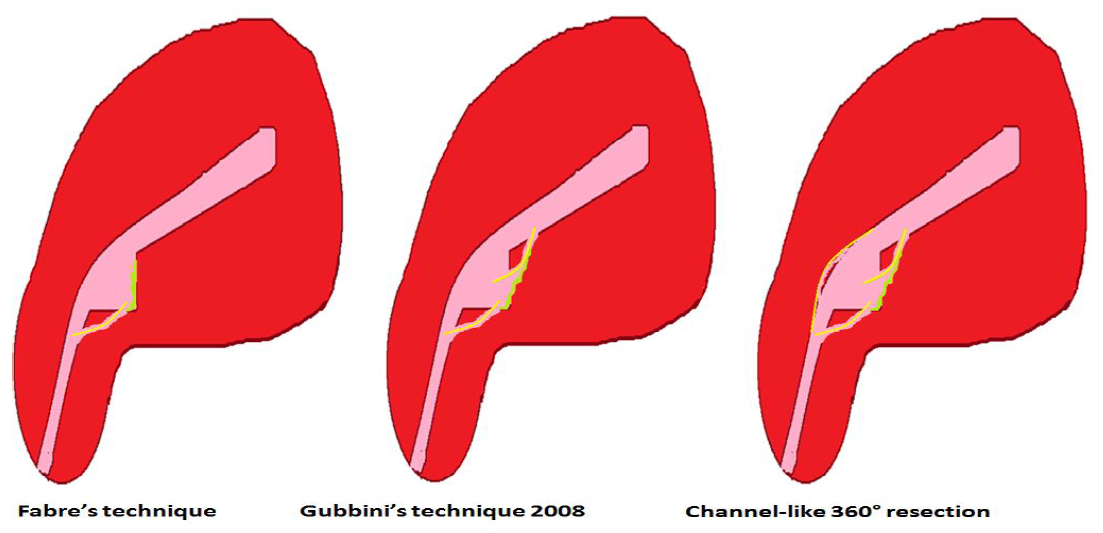
The hysteroscopic management of isthmocele exclusively targets this particular simple triangular pouch with a minimum residual myometrium of 2.5-3 mm and consists of coagulating its roof after resection of either the distal edge alone in Fabre’s technique (8, 11); the distal and proximal edge in Gubbini’s classic technique 2008 (10) or channel-like 360° resection of the whole canal in the latest proposed techniques (12) (Fig 2). Resection of the hard fibrous tissue is thought to allow subsequent remodeling of the residual myometrium under improved uterine contractility. However, to date there is no comparative study to prove the superiority of any technique over the other.
More importantly, this simple triangle-shaped isthmocele only addresses the simplest form of CSD which, time passing, reveals various shapes and complex mechanisms needing to be acknowledged and addressed in the surgical treatment.
Complex isthmocele: atypical anatomy – heterogeneous shapes and branching
One of the problems resulting from the absence of pertinent consensual definitions of isthmocele is experienced when dealing with cases of CSD which are different from the above-mentioned simple triangle-shaped ones.
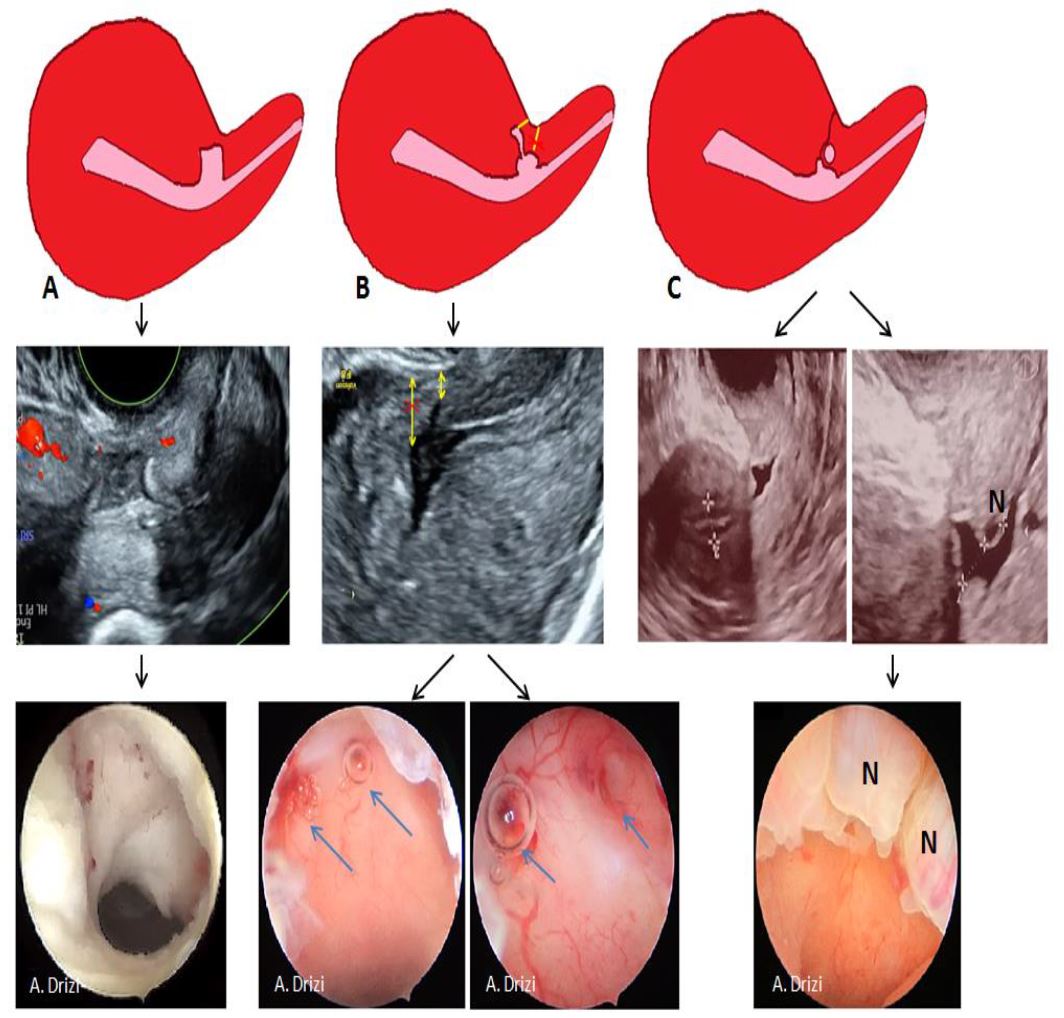
In fact, all practitioners usually encounter morphological differences in real life practice. Ultrasonography experts have already proposed a more consistent classification taking into consideration the heterogeneous shapes and branches of the USDs, which do not always happen to be wedge-shaped but at times mimicking a round, square or even a cribriform area (8,9,13).
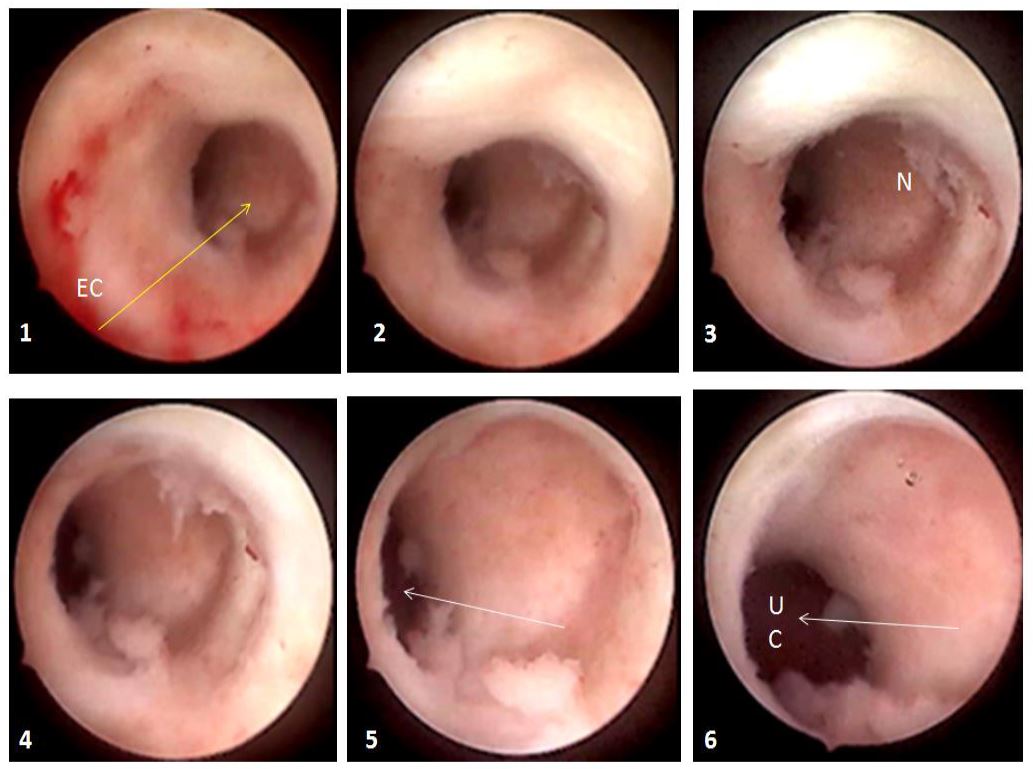
The presence of branches in the roof of the isthmocele is particularly important to note as it classifies the CSD among the complex ones and as it decreases the real residual myometrium which always needs to be measured at the site where it is the thinnest (9, 13) (Fig 3). The presence of Nabothian cysts at the level of the uterine scar is another common anatomical finding which can be misleading to over-diagnosis of CSD whereas it is a differential diagnosis (8) (fig 3).
However, whether the USD is anatomically complex and/or associated with Nabothian cysts, its hysteroscopic management remains unclear as there are to dates no guidelines to specify how to approach them.
The presence of branches has already been acknowledged by the ultrasonographers as modifying the level at which the residual myometrium needs to be measured: rather from the top of the branch and not from the roof of the niche itself (9, 13). During diagnostic hysteroscopy, attention should be given to the movement of air bubbles within the niche, as they tend to go through the branches’ orifices (fig 3).
Still, the question of how to optimally resect an anatomically complex isthmocele via hysteroscopy has never been addressed in a study. Moreover, the anatomical varieties of operated niches are never detailed in the available studies. And just as importantly, the complexity of CSD is not only anatomical but seems to involve additional non anatomical mechanisms which requiring closer attention.
Complex mechanisms in isthmocele other than blood collection: vascular, inflammatory, polypoid, endometriotic and myometrial changes
In addition to the classical pathogenic mechanism of menstrual blood trapped in and distending the CSD, in-situ provenance of the bleeding has already been mentioned by some authors.
Among the etiopathogenic factors of isthmocele, cervical location explains local mucus-like secretion thus perturbing the healing process and causing local inflammation (4). The latter is well known to cause AUB, also because of the resulting abnormal vascularization around the scar (10).
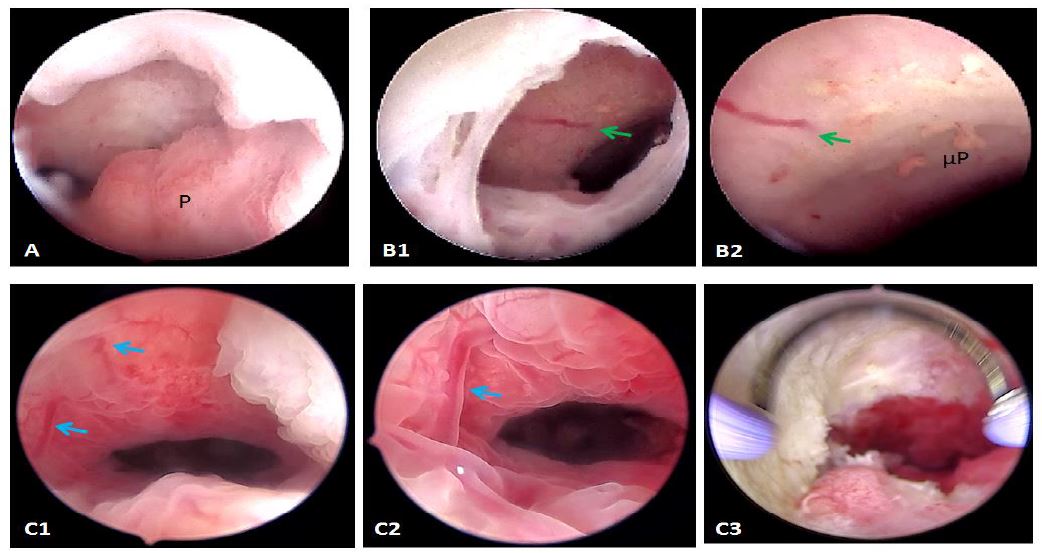
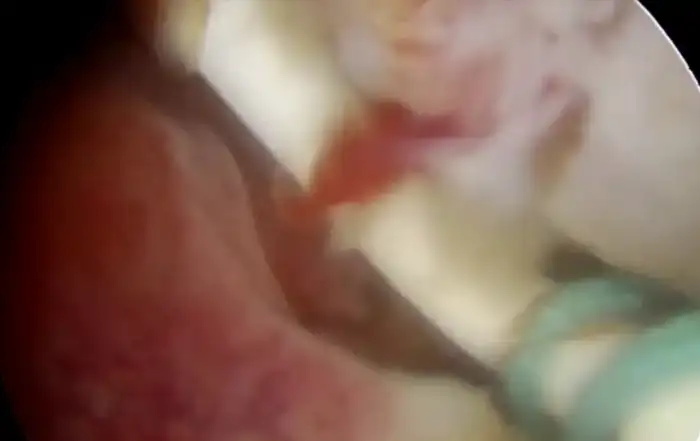
Chronic inflammation is already known for being the optimal ground for proliferative processes in all branches of medicine. Its role as a risk factor for the development of endometrial polyps has already been documented in the literature (14). Although its association with symptomatic isthmocele has never been assessed to date, polyps are not a rare finding in our practice. In-situ bleeding from abnormal local vessels of the niche is not rarely encountered in our practice either (Fig 4).
In addition to inflammatory, polypoid and vascular anomalies, endometriosis has also been reported as a non-negligible intraoperative finding in patients with symptomatic isthmocele, with a special focus on the laparoscopic approach, especially in women with secondary infertility and dysmenorrhea (15,16). Isthmocele is thus a recent condition still requiring more investigations.
Myometrial changes could be thought of as the resulting altered muscular contractility due to the presence of a hard fibrous scar tissue within an elastic dynamic muscle, hence perturbing its contractility. This has been described as one of the factors causing abnormal evacuation of menstrual blood outside the uterus during menses (10). Regardless of that, isthmocele being considered as a static distended hernia passively collecting blood can also be questioned, especially as the uterus is a muscular dynamic organ.
Complex isthmocele: a dynamic entity? The Aggarwal valve
In all the available published data on isthmocele, the defect is always presented as a distended static space where secretions and blood can be collected and subsequently released in the form of post menstrual bloody discharge. The only dynamic facet of CSD is the myometrial contractility which is reportedly altered by the presence of scar tissue.
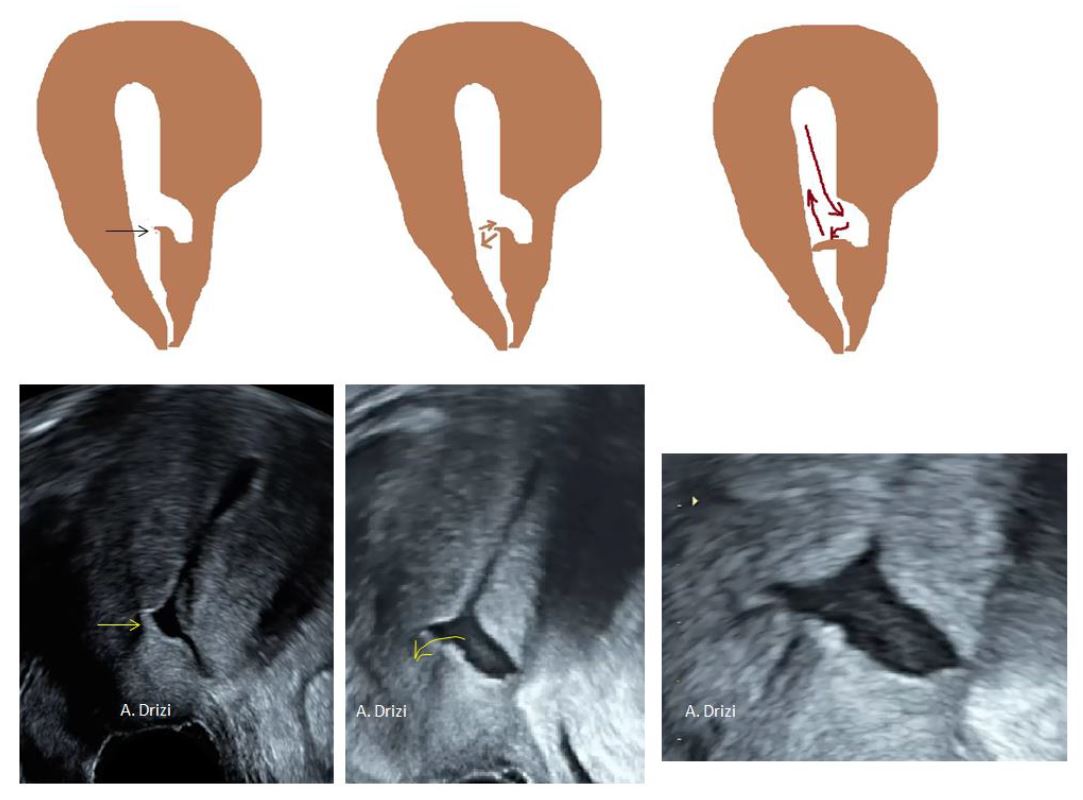
However, an Indian author has recently reported cases where the distal edge of the niche is acting like a valve under myometrial contractility thus closing the cervical canal and explaining cryptomenorrhea. This is explained by the enlargement of the pouch and the consequent thinning of the wall both towards serosa and cervical canal which would result in a distal edge taking the form of a fibrous-mucosal fold oriented towards the canal and which, just like a valve, would close the passage to the canal when pushed down by the blood flow, thus decreasing evacuation of menses (17) (fig 5).
This dynamic pathogenic mechanism is indeed observed at ultrasound in some cases where the movement of the distal edge of isthmocele is filmed during uterine contractions (Fig 5). In hysteroscopy, it is difficult to observe this phenomenon as the distending medium movement goes from the cervix to the cavity in order to open the virtual spaces.
Therefore, although laparoscopic resection was the technique demonstrated for this mechanism, it appears that hysteroscopic resection is also effective as all the usual techniques (10-12) target the distal edge of the niche, hence allowing suppression of the valve-like acting system. Of course, more studies are needed to explore in depth this potentially path-breaking dynamic mechanism.
Isthmocele: rather a complex multifactorial entity
Given the lack of standard consensus regarding classification and management of isthmocele, it is of utmost importance to remain careful to the various mechanisms in order to identify this or those involved in each patient, so as to thoughtfully target them whenever possible. Moreover, reporting the diagnostic details of the operated cases is the only warrant of more pertinent studies leading to a better understanding of the condition in its diverse facets as well as to more optimized treatment strategies depending on the variety’s type. Consequently, a constant attention to details during diagnostic hysteroscopy is of high importance, as it can reveal additional anomalies which are difficult to judge at ultrasound such as angulations and twisted path of the cervico-isthmic canal (Fig 6).
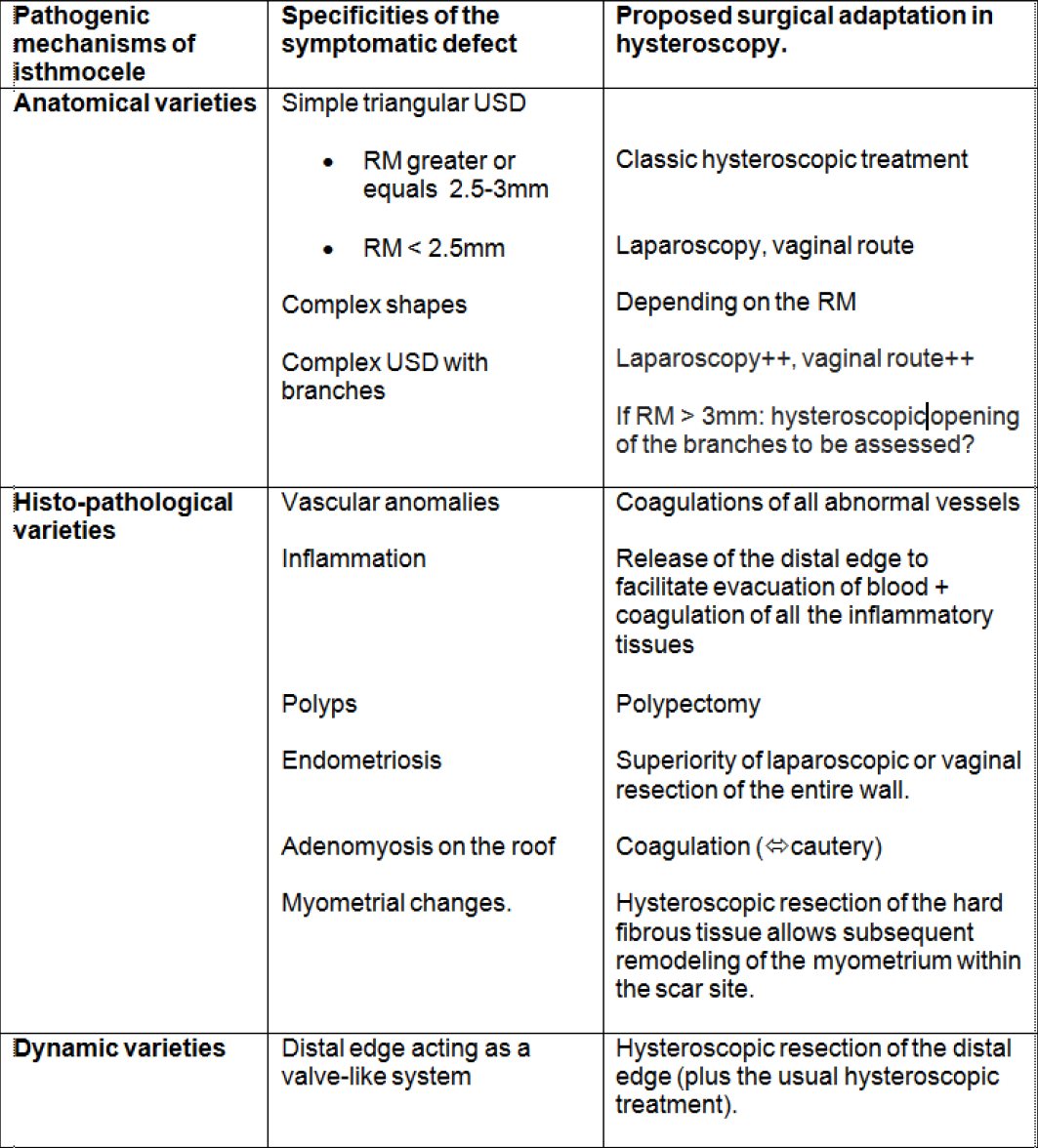
In table 2, a diagram is proposed to summarize the different classes of isthmocele overviewed in this article, as well as the therapeutic approach likely to tackle the specific pathogenic mechanism in each one.
Conclusion
With the progress of imaging techniques, hysteroscopy does not need to be performed in the unique purpose of posing the diagnosis of a symptomatic isthmocele but rather as a mandatory first step within the same operative session. Despite the lack of understanding surrounding the isthmocele as an anatomo-clinical entity, it appears as a multifactorial condition where heterogeneous mechanisms can be impaired.
Its appears as one area where ultrasonography and diagnostic hysteroscopy are more advanced than operative hysteroscopy which, in all cases, can only be improved if taking into consideration the different varieties.
It is of utmost importance to define more pertinent classifications of the condition separating the simple classic forms from the complex ones as this could impact the choice for the optimal surgical treatment. Therefore, and in our practice of ultrasound and diagnostic hysteroscopy, we propose to classify isthmoceles in simple and complex ones. Complexity can be anatomical, histo-pathological and dynamic. This would provide a more accurate basis for the assessment of the surgical treatment.
References
Table 1. Main risk factors associated with isthmocele
Figure 1. Correlations between 2D ultrasound of isthmocele and diagnostic hysteroscopy. D: distal edge; P: proximal edge; B: basis; blue arrow: depth of the niche; RM: residual myometrium. Images by A. Drizi.
Figure 2. The hysteroscopic techniques of isthmocele treatment
Figure 3. Complex anatomy of isthmocele with sonographic and hysteroscopic correlations. A: square-shaped niche; B: branch on the top of the niche with air bubbles passage at hysteroscopy through the orifices; C: Nabothian cyst near isthmocele. Yellow arrow: residual myometrium; Blue arrow: the orifice on the top of the niche leading to the branch with air bubbles passage (complex isthmocele). N: Nabothian cyst. Images by A. Drizi.
Figure 4. Complex mechanisms involved in the isthmocele’s pathogenesis. A: Polyp (P) inside the niche; B1: Spontaneous bleeding from a vessel on the roof of the niche (green arrow); B2: closer view with inflammatory patterns like micropolyps (μP); C1: ectatic vessels in a symptomatic niche (Blue arrows); C2: Closer view; C3: after resection and coagulation. Images by A. Drizi.
Figure 5. Demonstration of the valve mechanism by the distal edge and correlation with ultrasound at increasing magnification (black and yellow arrows). The flow of the blood closes the valve resulting in decreased evacuation of menses and causing more distention. Images by A. Drizi.
Figure 6. Lateral angulation at the level of the niche (N). 1-6: progression from the endocervical canal (EC) until the entry of the uterine cavity (UC). Yellow arrow: orientation of the canal from the right to the left of the patient; white arrow: orientation of the cavity from the left to the right of the patient, resulting in an angulation (images by A. Drizi).
Table 2. Summary of the different classes of isthmocele overviewed in this article, as well as the therapeutic approach likely to tackle the specific pathogenic mechanism in each case.