Authors / metadata
DOI: 10.36205/trocar1.2021004
Abstract
Background: Cesarean section scar pregnancy is one of the rarest forms of ectopic pregnancy where the gestational sac is fully or partially implanted within the scar caused by a previous caesarean section. Its incidence is on the rise due to the increasing rate of cesarean sections (CS) and also to the increasing awareness and the better ultrasound diagnosis.
Case presentation: A 28-year-old woman G3P2 with a history of 2 cesarean deliveries who was diagnosed by ultrasound scan at 8 weeks gestation with cesarean scar ectopic pregnancy that was confirmed by pelvic magnetic resonance imaging (MRI). At 4 weeks the patient had a pelvic ultrasound suggested an intrauterine pregnancy with a gestational sac visualized in the lower uterine segment suggesting a cervical stage of miscarriage. Surgical management was opted for a combination of laparoscopy and hysteroscopy. A ligation of bilateral ascending uterine artery ligation was performed.
Conclusion: Cesarean section scar pregnancy (CSP) is rare but life threating complication. It should be diagnosed and treated early because of the increased risk of massive hemorrhage or uterine rupture that engages the vital prognosis and the functional prognosis.
Corresponding author: Hind Ennasser E-mail address: mailto:hindennasser@gmail.com
Introduction
Cesarean section scar pregnancy is a rare form of ectopic pregnancy that increased in recent years due to the parallel increase of CS. It’s a complex iatrogenic pathology defined as the implantation of the gestational sac in the myometrium at the site of a previous CS [1]. The first case was reported by Larsen and Solomon in 1978[2]. At early pregnancy it can be confused with a cervical stage of miscarriage or cervical pregnancy. Because of the dangerous complications such as uterine rupture, uncontrollable bleeding, and hemorrhage into the abdominal cavity; an early diagnosis and therapy are necessary to prevent the development of severe complications [3]. Here we report a case of this uncommon presentation of ectopic pregnancy with laparoscopic and hysteroscopic management in our department, this is the first case in the country managed by laparoscopy.
Case report
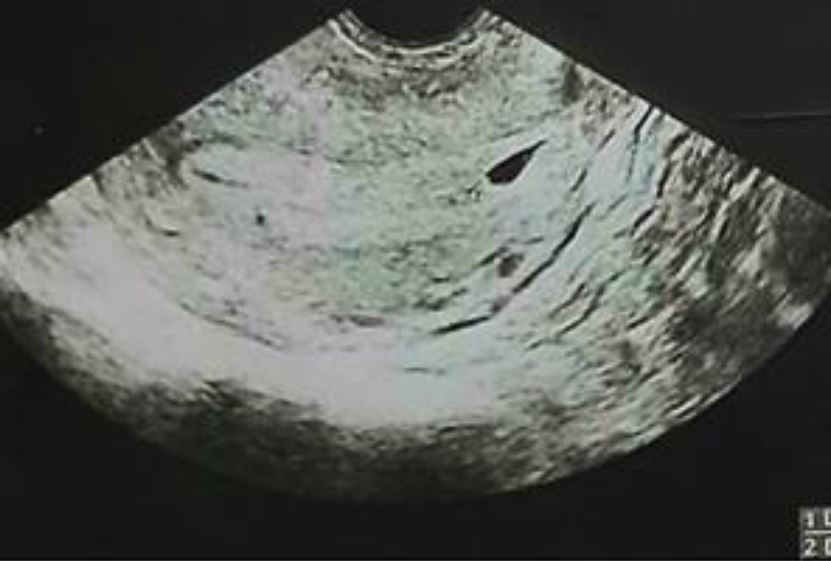
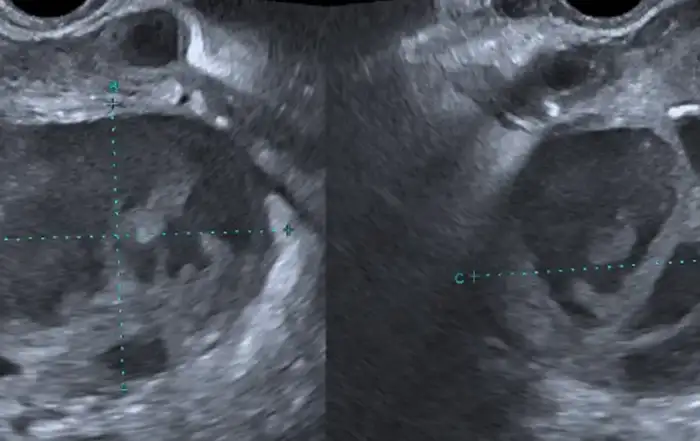
A 28 years old woman G3P2 with a history of 2 cesarean deliveries in the past, presented to the emergency department for minimal subacute intermittent blackish abnormal uterine bleeding at eight weeks and three days of amenorrhea without pelvic pain. Her most recent pregnancy was 3 years prior to the consultation. At 4 weeks and 4 days the patient did have a pelvic ultrasound suggesting an intrauterine pregnancy with a gestational sac visualized in the lower uterine segment revealing a non-viable detached intrauterine pregnancy (fig1) with a serum concentration of human chorionic gonadotropin (HCG) at 8306 mUI/ml.
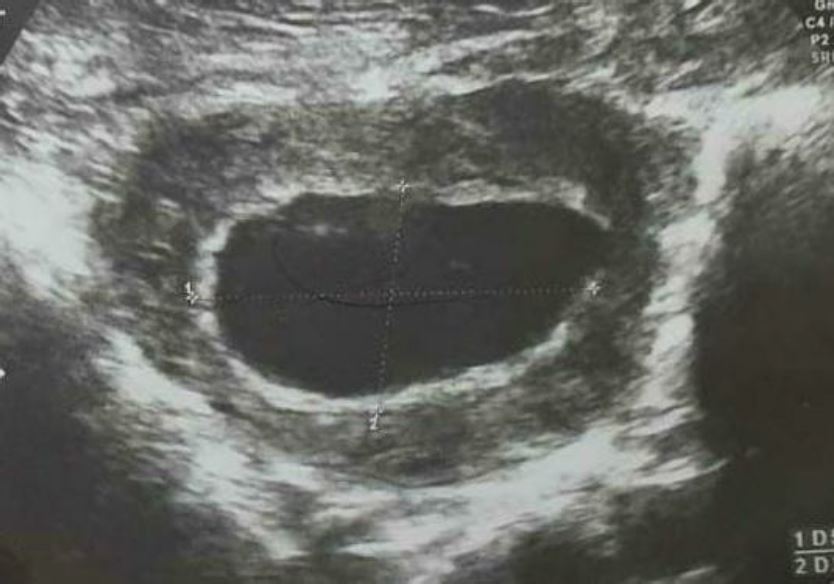
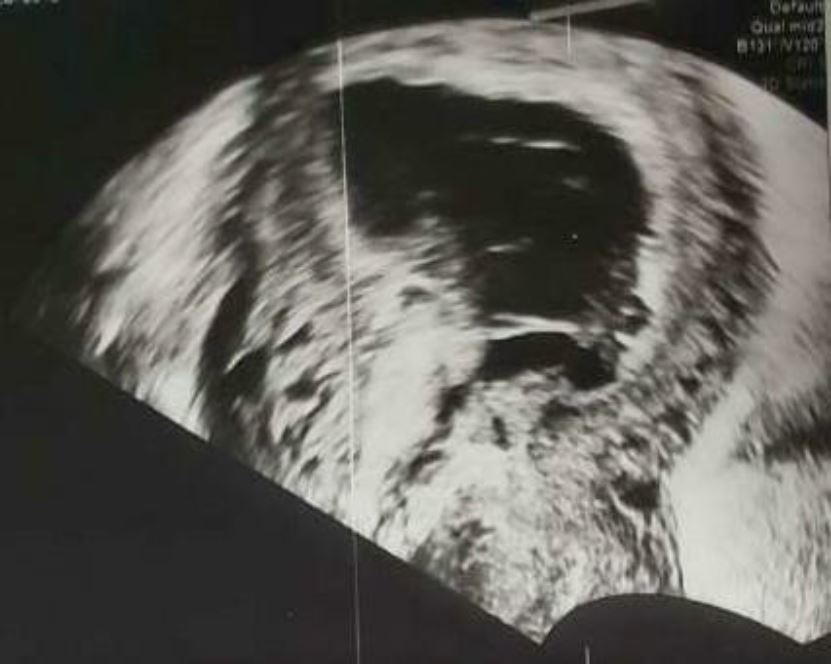
On clinical examination, the hemodynamic parameters were stable. The abdomen was supple at deep palpation. At Gynecological examination the only notable finding was a minimal blackish bleeding originating from a closed cervix at speculum. A pelvic and endo-vaginal ultrasound performed in the emergency department showed a low implanted intra-uterine sac of 52/28 mm at the level of prior cesarean scar in the lower uterine segment with a small rim of myometrium visible anterior to the gestational sac. The gestational sac was communicating with the endometrial cavity, whilst being located in the lower uterine segment of uterus. The cervical canal was closed and empty with no intraperitoneal fluid was noted (fig2, 3).
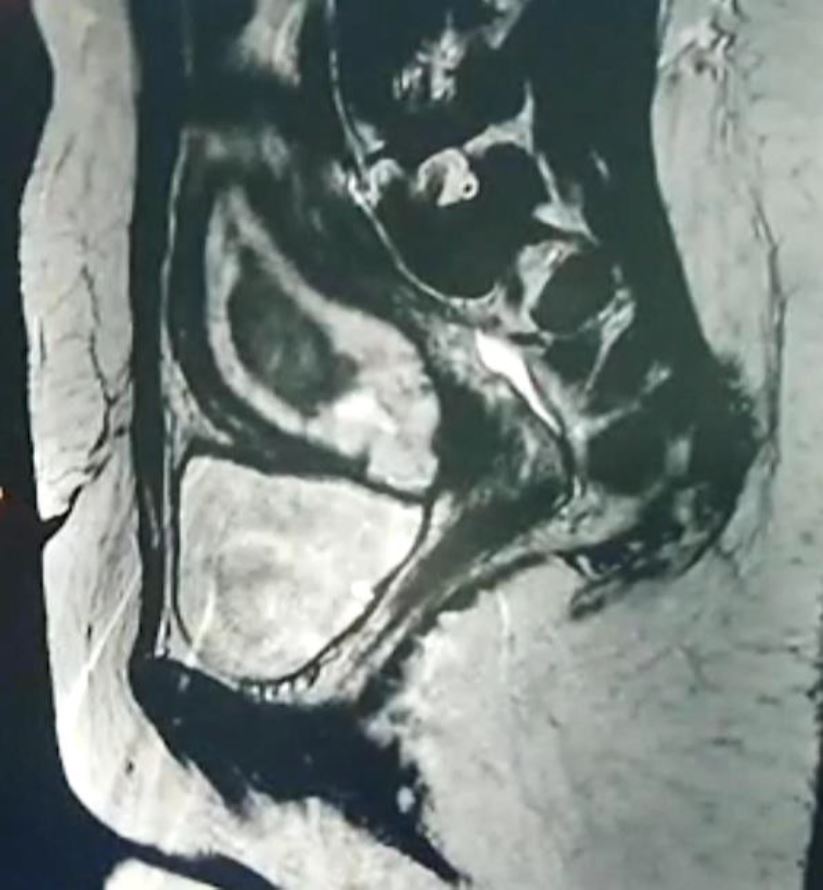
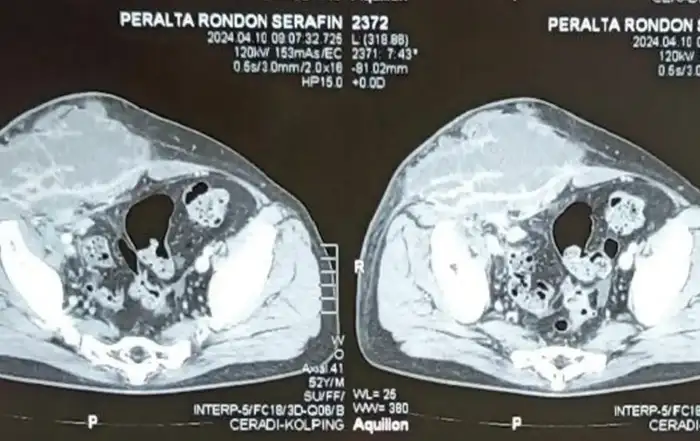
The serum HCG concentration was 40540 mUI/ml where the hemoglobin and hematocrit level were normal, 48 hours later serum HCG was at 53280 mUI/ml. A MRI revealed a gestational sac embedded in the hysterotomy scar, coming down to the serosa without the interposition of the myometrium, lateral on the right side (fig4). The diagnosis of caesarean section scar pregnancy was retained. A decision was made to proceed with a surgical management in the form of a laparoscopic resection of the ectopic pregnancy after hysteroscopic evaluation.
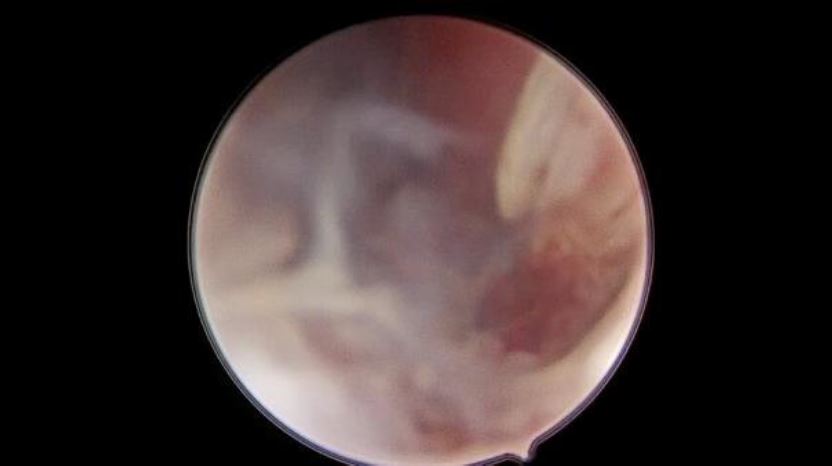
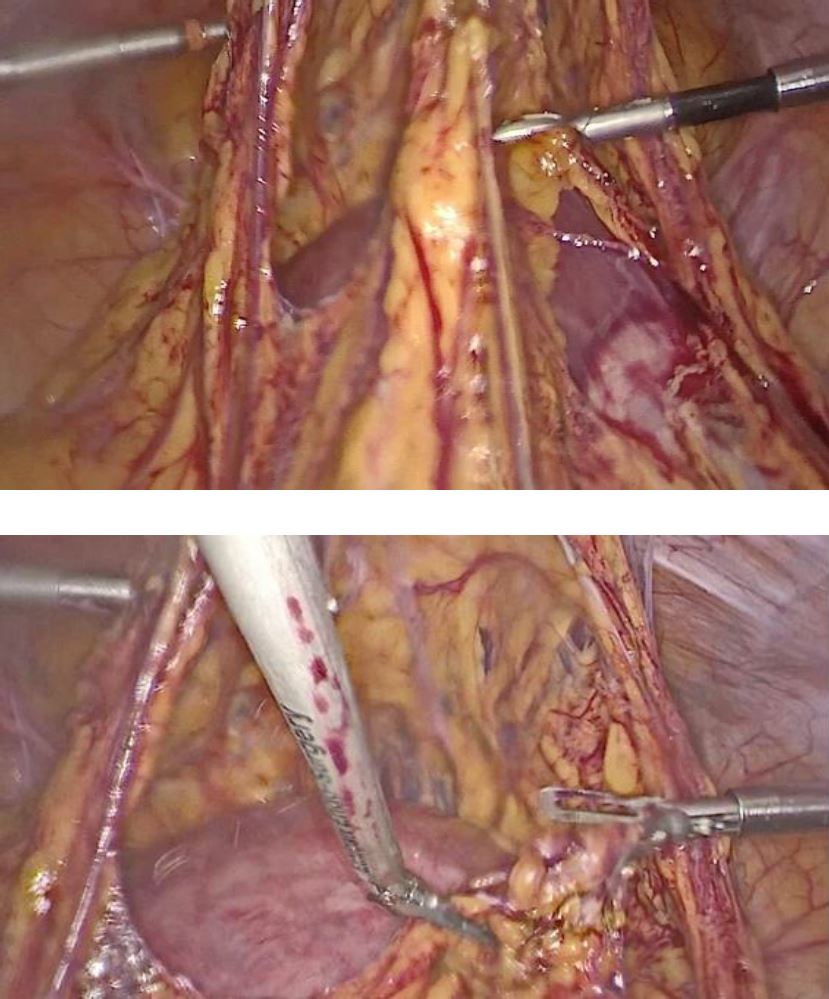
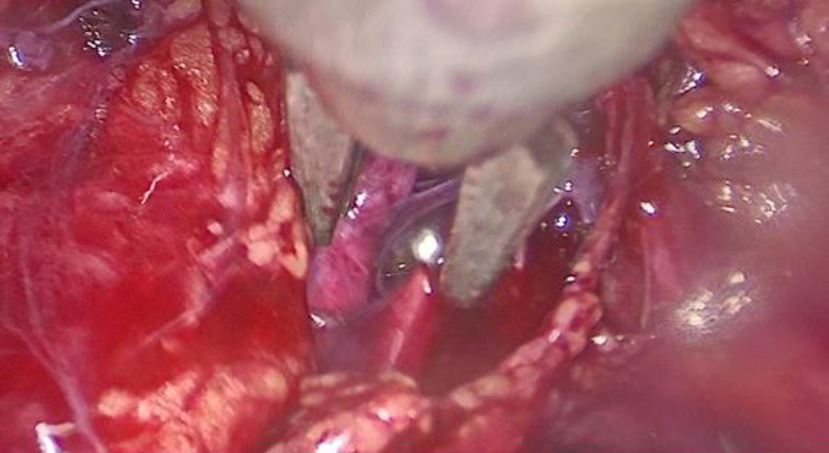
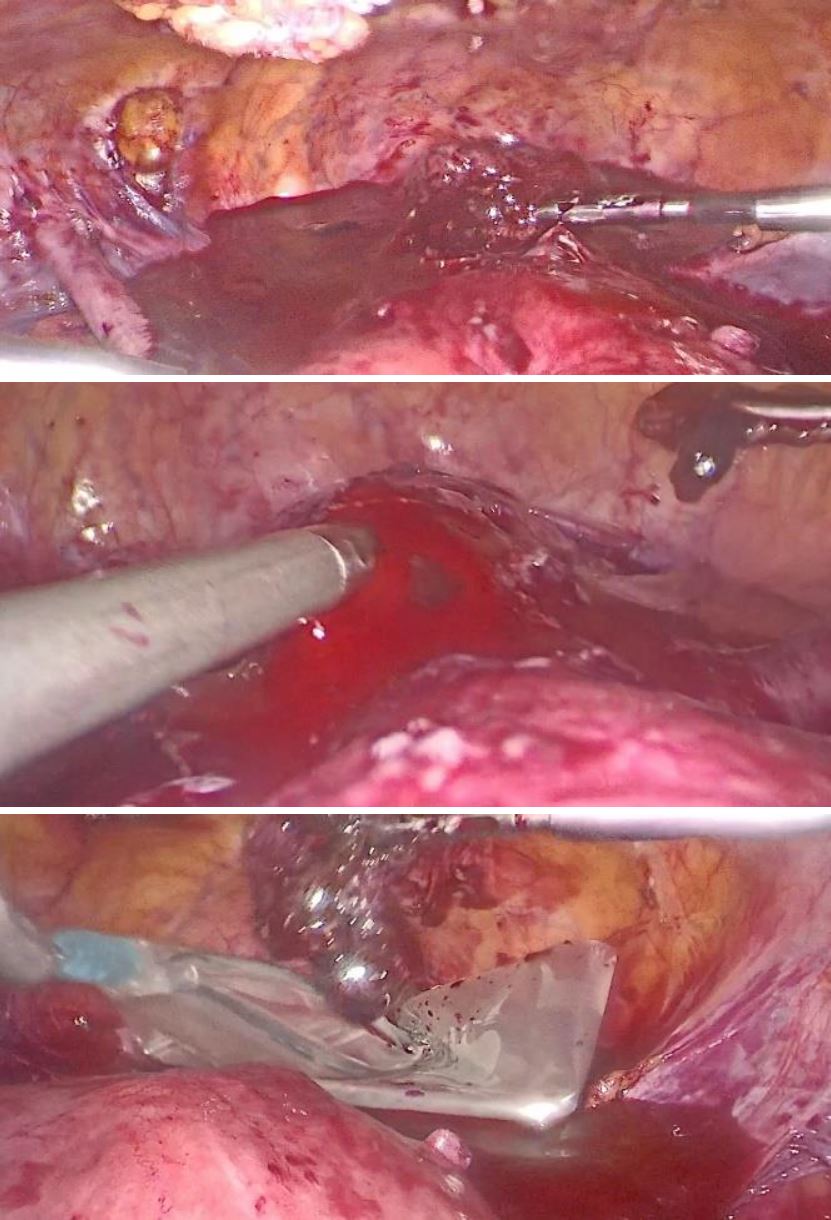
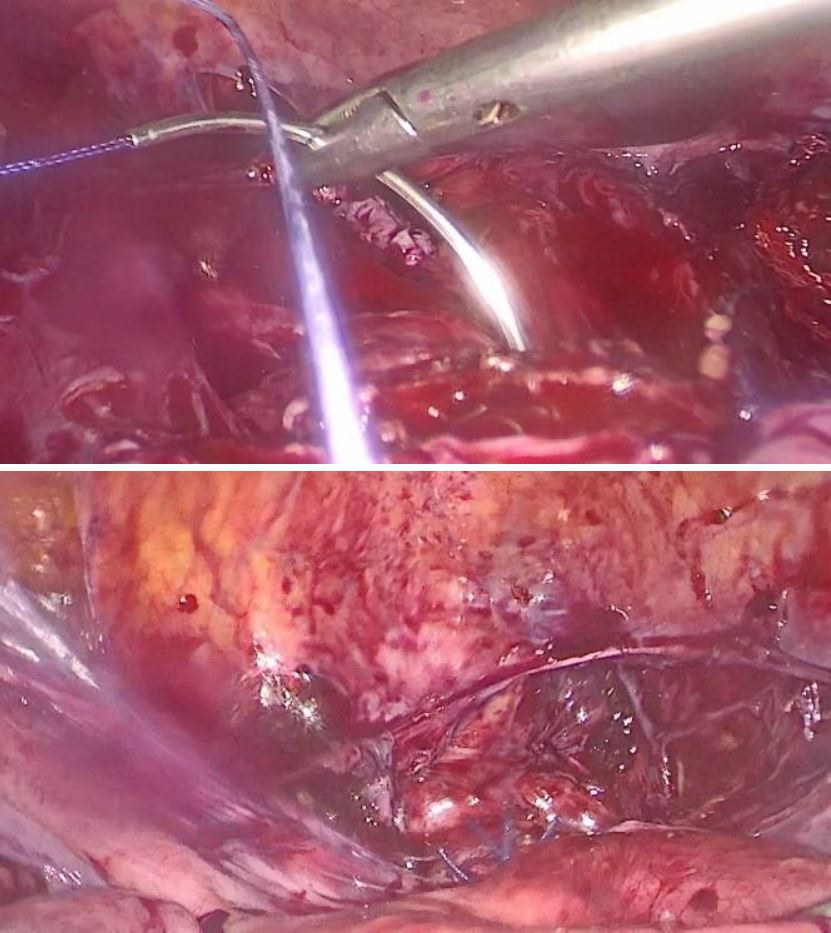
Under general anesthesia a diagnostic hysteroscopy was performed first reveling the attachment of chorionic tissues around the isthmus (fig5). Then a laparoscopic exploration did follow. The omentum was densely adherent to the uterine surface, an adhesiolysis was done (fig6). A bilateral uterine artery ligation was performed for security reasons (fig7). The bladder was dissected down to expose vesico-vaginal space, a protrusion into the anterior wall of the uterus at the uterine isthmus was found (isthmocele) (fig8).
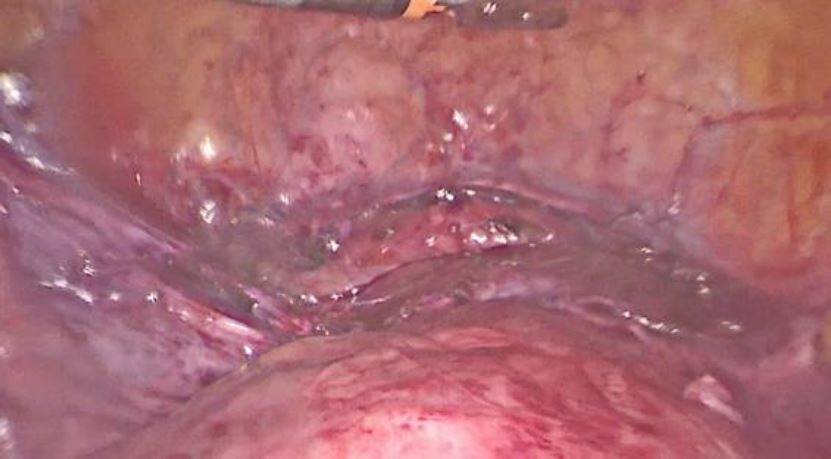
An incision over the bulge was done then the trophoblastic tissue was removed and placed in an extractor sac (fig9). A suction curettage was performed. Interrupted Vicryl-0 sutures were used for closure of the defect (fig10). The specimen was sent for histopathological examination. The patient was discharged two days after the surgery.
She was informed by the risk of recurrence of CSP, uterine rupture and placenta accreta for future pregnancies. Contraceptive advice was also given. Two weeks later at follow-up, the patient’s HCG was negative. The ultrasound examination revealed an empty uterine cavity.
Discussion
As one of the rarest forms of ectopic pregnancy, CSP is reported to occur in 1/1800 to 1/2216 of pregnancies, and it constitutes 6.1% of all ectopic pregnancies with a history of at least one cesarean delivery [4]. This kind of ectopic pregnancy is increasing in frequency, due to the increase in the number of caesareans in recent years.
Little is known about the mechanism and physiopathology of CSP. The mechanism suggested is the implanting blastocyst to invade the scar through a microscopic tract that develops from the trauma of an earlier cesarean scar [5]. Vial et al. suggested that there are two types of CSP. The first one results in the implantation of the fertilized egg in the scar and it development in the direction of the cervical isthmus and uterine cavity. This gives a chance for live birth, but the risk of massive bleeding from the implantation site is very high. The second type of CSP is represented by a deep implantation in a damaged cesarean scar and the development of a pregnancy leading to uterine rupture in the first trimester of gestation [6].
The diagnosis of pregnancy in a cesarean section scar is often made in the first trimester. In a review of 57 patients with CSP 38.6% presented with vaginal bleeding, and 24.6% presented with abdominal pain, as for a 36.8% of the patients were asymptomatic being incidentally detected by routine ultra-sonographic examination [7]. In an advanced stage, a CSP may result in uterine rupture leading to massive haemorrhage, haemoperitoneum and haemorrhagic shock.
Endo vaginal ultrasound allows an early diagnosis based on criteria established by Vial in 2000 represented in an empty uterine cavity and empty cervical canal, placenta or a gestational sac embedded in the scar of a previous caesarean section on sagittal section of the uterus [8]. There are also indirect ultrasound signs, such as decreased myometrial thickness between the gestational sac and the bladder and a circular blood flow surrounding the sac seen by color Doppler [9].
An MRI may provide additional confirmation of the ultrasound findings and specify the depth of trophoblastic invasion in the myometrium and the potential involvement of the serosa or bladder [10].
From the studies reviewed, no universal treatment guidelines have been established yet. There are several variables to consider before recommending a treatment option as there are the haemodynamic stability, desire to preserve fertility and acceptability of prolonged follow up. Treatment modalities are either medical or surgical and are sometimes combined.
The medical approach consists of an administration of methotrexate through systemic route, or local administration (under ultrasound or laparoscopic-guidance) or a combination of the two [11, 12]. It takes about 4 to 6 weeks to normalize the HCG [9].
The surgical approach which is often conservative consist on removing the pregnancy and a repair of the uterine defect by laparotomy or laparoscopy., then the HCG normalization is reached in one to two weeks [13].
Because of the possibility of massive haemorrhage a preventive hemostasis by ligation of the uterine or hypogastric arteries may be necessary. Interventional radiology techniques like uterine artery embolization (UAE) can also be used preoperatively to decrease the risk of hemorrhage hysteroscopy [9]. UAE or vascular ligation does not appear to reflect fertility or the obstetrical prognosis following the patients [14].
The operative hysteroscopy and suction curettage have been described in various studies of CSP with a muscular layer ≥3mm [15]. It consists of the aspiration of the conceptus, haemostasis can be achieved with electro coagulation. A balloon catheter may be placed postoperatively for compression haemostasis. In a case series by Pan et al, hysteroscopy was used in conjunction with laparoscopy for patients whose muscular layer was< 3mm to avoid the risk of uterine perforation and bladder injury [15].
For future pregnancy the risk of recurrence is estimated at 5% [16]. If further pregnancies are planned a delay of 12 to 24 months between pregnancy on cesarean section scar and future pregnancy is recommended [9]. In a case series by Wei et al the reproductive outcomes for women with history of CSP were followed up, spontaneous pregnancy rate was 74.0% and the recurrence rate was 14.3% [17]. Also, Ben Nagi et Al shows that reproductive outcomes following Caesarean scar ectopic pregnancies are favorable, 83% of women were able to achieve subsequent pregnancies with a median time interval between previous scar ectopics and new conception of 5.3 months (range 1–48 months). 5% had a recurrent CSP [18].
Conclusion
CSP is an uncommon form of ectopic pregnancy that can result into life threatening complications if not diagnosed and managed early. In our case there was a high clinical suspicion for a CSP in a patient with a history of cesarean deliveries presenting with first trimester bleeding.
Diagnosis and management of CSP could sometimes be challenging and requires a multidisciplinary approach. Laparoscopic management is considered as a safe option with shorter operation – and hospitalization time, less intraoperative bleeding, faster recovery.
In order to reduce the incidence of this iatrogenic entity, the reduction in the number of primary CS performed without medical indication is necessary.
Abbreviations
Authors’ contributions
Ahmed Mimouni performed the surgery as a main surgeon. The manuscript was prepared by Hind Ennasser. Imane Skiker analyzed and interpreted the MRI. Hanane Saadi and Hafsa Taheri were the second and third surgeon.
All authors read and approved the final manuscript.
Funding: None of the author received any funding for this study.
Ethics approval and consent to participate.
The privacy of the patient was considered, and the manuscript does not include any identifying information.
Consent for publication: Informed consent for publication of the patient’s clinical data and the accompanying/ images was obtained from the patient.
Competing interests: The authors declare that they have no competing interests.
Sources / references
Figure 1: Sagittal Transabdominal ultrasound shows the gestational sac in the lower uterine segment
Figure 2: transversal transabdominal pelvic ultrasound imaging shows intra-uterine gestational sac of 52/28 mm
Figure 3: Sagittal endovaginal ultrasound demonstrating the characteristics of cesarean scar ectopic pregnancy : low lying gestational sac with anterior myometrial thickness , absence of cervical involvement
Figure 4: Magnetic resonance image shows a gestational sac implanted in the anterior wall of the uterus, and protruded into the uterine cavity.
Figure 5: hysteroscopic view of trophoblastic tissue
Figure 6: laparoscopic finding of the adherences of omentum to the uterine surface processes of adhesiolysis
Figure 7: ligation of right and left uterine artery using clips
Figure 8: visualization of the isthmocele
Figure 9: Incision over the bulge, excision of the trophoblastic tissue and it placement in extractor sac
Figure 10: the closure of the defect using interrupted Vicryl-0 sutures