Authors / metadata
DOI: 10.36205/trocar1.2021002
Abstract
Background: Adenomyosis is a common condition that is often associated with poor reproductive outcome. Repeated implantation failure in in vitro fertilization (IVF) cycles can result from impaired implantation caused by adenomyosis. The evidence regarding the role of long-term suppression (LTS) with gonadotropin-releasing hormone agonists (GNRHa) prior to embryo transfer (ET) in cases of adenomyosis is limited and conflicting.
Aim: The aim of this study was to assess the efficacy of long-term GNRHa therapy on livebirth rates in women with adenomyosis and infertility.
Design: The following case series includes 15 women with infertility and known adenomyosis undergoing in vitro fertilisation (IVF) that underwent at least three months downregulation with GNRHa prior to ET. Outcomes were compared to previous cycles performed without LTS.
Results: LTS with GNRHa was given in 16 cases (94.1%) prior to ET. In one case (5.9%) 6 months suppression was given. The majority of these patients had previous unsuccessful IVF cycles prior to LTS protocol with GnHRa. 17 embryo transfers (16 frozen, 1 fresh from donor) following LTS protocol resulted in 10 liveborn deliveries at term (58.8%) vs. a live birth rate of only 7.7% per ET without LTS (26 embryo transfers resulting in 2 live births; P<0.0001). Only three women who underwent long term GnRHa downregulation had no successful embryo transfers.
Conclusion: Our successful outcomes support the use of LTS with GnRHa to improve reproductive outcomes.
Corresponding author: Dr Catherine Schepisi catherineschepisi@gmail.com
Introduction
Adenomyosis is a benign estrogen dependent disease whereby ectopic endometrial glands or stroma are found within the myometrium, surrounded by myometrial smooth muscle cell hyperplasia and hypertrophy [1-3]. It can be diffuse or focal depending on extent of uterine spread [4, 5]. Women with adenomyosis typically present with dysmenorrhea, heavy menstrual bleeding or infertility, however a large proportion can be asymptomatic [3]. Therefore, true prevalence is unknown, with large variations between 5-70% cited within the literature [3, 6, 7]. Previously thought to be a disease associated with older multiparous women, delayed fertility and advancements in imaging techniques have led to an increased diagnosis in younger women of reproductive age being investigated for infertility [8]. Adenomyosis has been associated with poor reproductive prognoses [9, 10]. Multiple factors have been implicated including impaired implantation and utero-tubal disruption [11, 12]. Of particular interest is the impact of poor endometrial receptivity on implantation, which has been suggested to be more significant than embryo quality [13]. Increasing evidence is emerging to support the role of long-term GnRHa downregulation in improved reproductive outcomes, especially when used in conjunction with IVF or intracytoplasmic sperm injection [7, 14-16]. The following case series details successful pregnancy outcomes following GnRHa down regulation prior to embryo transfer (ET), further supporting the existing evidence and theorises its main mechanism of action is by improving endometrial receptivity.
Materials and methods
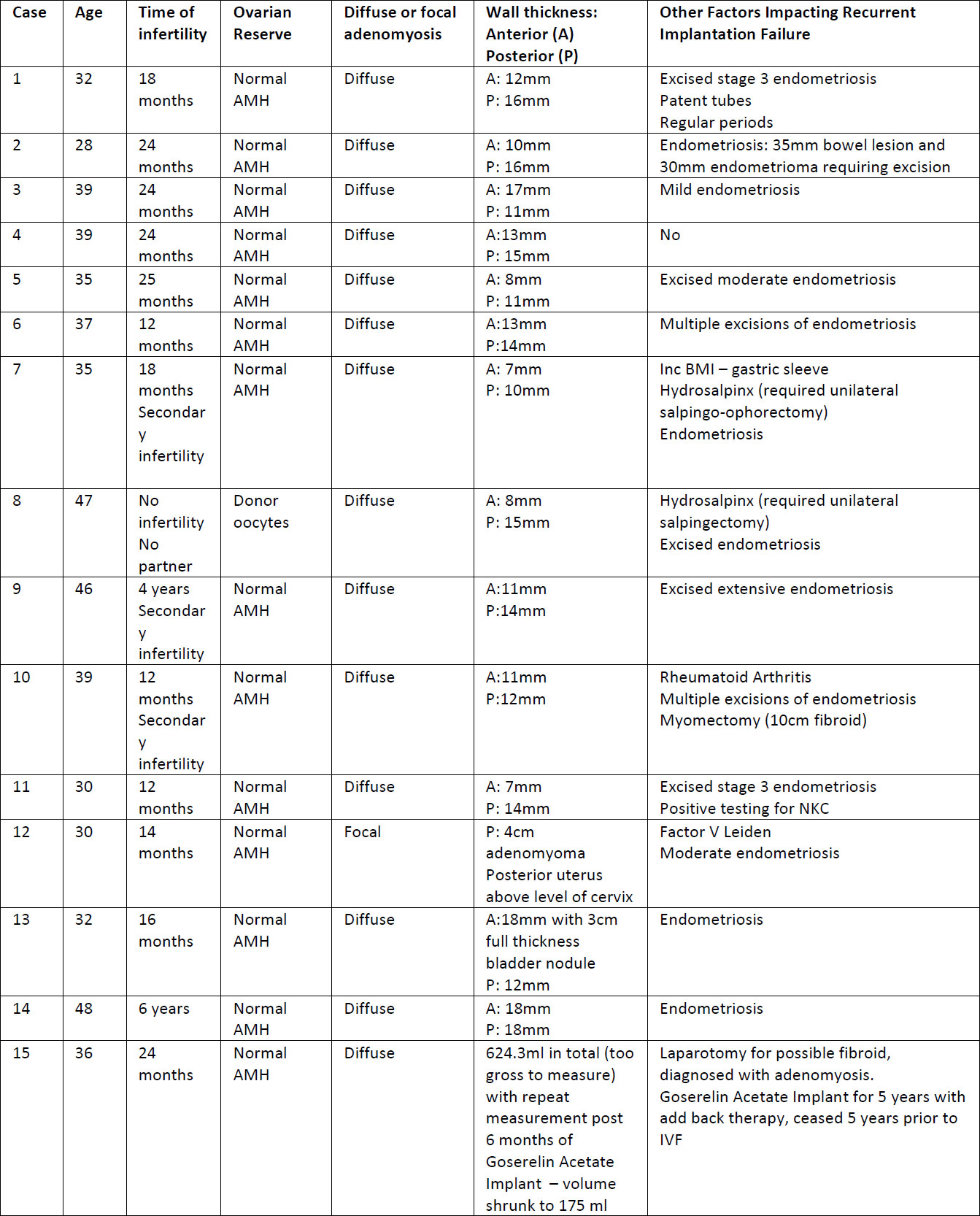
This is a retrospective case series that includes 15 patients diagnosed with adenomyosis and treated with long acting GnRHa prior to their IVF treatment. These patients were chosen from a population of patients attending a private IVF clinic. All women included required an ultrasound diagnosis of adenomyosis. Patients were treated with at least 3 months of Goserelin Acetate Implant (Zoladex®) as part of the IVF protocol prior to ET. The study was reviewed by the Epworth Healthcare ethical review board and registered as a quality assurance study. Data was retrospectively collated from patient files and included patient demographics, previous obstetric history, prior fertility treatment and outcomes as well as significant past medical history. Data were analysed to assess the effect of long-term GnRHa downregulation on reproductive outcomes. Other factors impacting fertility were also recorded for each patient (See Table 1).
Data analysis was performed using SPSS Statistics, version 11. Descriptive characteristics of data are presented as median and interquartile range. Test of statistical significance for categorical variables was done using Pearson’s chi-square test and T-Test for non-categorical variables. Statistical significance was set at a p value <0.05.
Results
received long acting GnRHa down regulation were reviewed. The average age of the patients was 37.5 years (range 28-48, SD 6.5). Most of the patients had concurrent diagnosis of endometriosis (13 patients, 86.7%) that was also treated prior to the commencement of the current long-acting suppression (LTS) protocol, none of the patients had surgery immediately prior to the commencement of the LTS protocol. All of the endometriosis surgeries that were performed occurred prior to cycles with regular ET without LTS. Diffuse adenomyosis was reported in 14 of the women (93.3%) while one patient had focal adenomyosis. All patients were diagnosed using ultrasound scans that were performed by a certified obstetrics and gynecology ultrasound specialists. Further data regarding patient characteristics can be found in Table 1.
Three months of long-term suppression with Zoladex was given in 16 cases (94.1%) prior to ET (Table 2). In one case (5.9%) 6 months suppression was given due to significant abnormal uterine bleeding. Frozen embryos were transferred in 16 (94.1%) patients after long term suppression (LTS) and a fresh embryo was transferred in one case (5.9%). Embryo transfers without LTS protocol included 15(57.7%) frozen embryos and 11 (42.3%) fresh embryos.
The majority of these patients had previous unsuccessful IVF cycles prior to LTS protocol with GnRHa down regulation. All patients had a single embryo transfer in all cycles. 17 embryo transfers (16 frozen, 1 fresh from donor) following LTS protocol resulted in 10 liveborn deliveries at term (58.8%) vs. a live birth rate of only 7.7% per ET without long term down regulation (26 embryo transfers resulting in 2 live births), this was statistically significant (P<0.0001). Only three women who underwent long term GnRHa downregulation had no successful embryo transfers. Total pregnancy rates were significantly better as well in the long-term suppression group (58.8% vs 19.2%, P=0.008 excluding chemical pregnancies, or 64.7% vs 26.9%, P=0.014 including chemical pregnancies).
Although most patients in this study had concurrent endometriosis, none of the LTS ET cycles were performed immediately following surgery for endometriosis. Furthermore, even though 9 patients underwent excision of endometriosis prior to other cycles, none of the patients had a live birth following the surgery (0%) while 6 of them (66.7%) had a live birth following LTS protocol. This superiority of LTS in cases of adenomyosis compared with endometriosis surgery was statistically significant (p=0.003).
Discussion
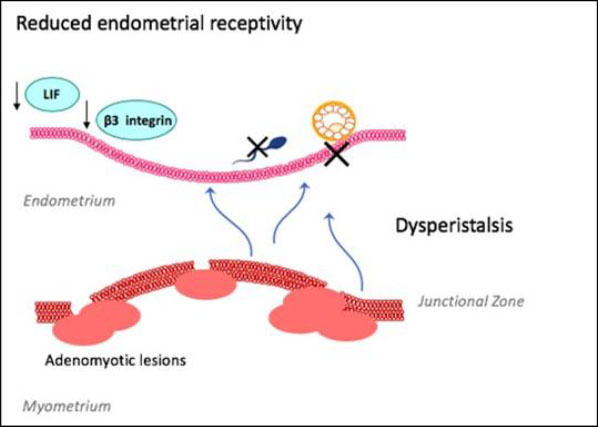
The pathogenesis of adenomyosis is not completely understood.[2] Adenomyosis is associated with a higher prevalence of recurrent pregnancy loss, failed assisted-reproductive treatment (ART) and poorer IVF reproductive outcomes [16-19]. Adenomyosis is suspected to impact fertility through a range of molecular mechanisms resulting in recurrent implantation failure (Figure 1). Adenomyosis related junctional zone disturbance causes dysperistalsis, impairing sperm transport and blastocyst implantation [12, 20, 21]. Hypoestrogenism, found in women with adenomyosis, perpetuates this dysperistalsis [22].
Impaired endometrial receptivity is also associated with implantation failure [23]. Adenomyosis has been associated with reduced endometrial receptivity markers, such as integrin ß3 and leukaemia-inhibiting factor, HOXA10 and HOXA11, which play critical roles in implantation as well as endometrial growth, differentiation and decidualisation[24, 25]. Pinopodes are morphological markers of endometrial receptivity seen on the endometrial surface at the time of implantation [25]. Decreased numbers and poorly formed pinopodes have been seen in human and mice studies with adenomyosis [25-27].
There however remains conflicting reviews in current literature regarding the impact of adenomyosis on infertility, with several authors concluding that cases with concurrent endometriosis confound and limit available evidence [16, 22, 28]. A 2014 meta-analysis found a 28% reduction in likelihood of clinical pregnancy in women with adenomyosis undergoing IVF or ICSI and suggests screening for adenomyosis prior to ART [15]. A retrospective cohort study of 213 women showed a significantly decreased rate of viable clinical pregnancies in women with adenomyosis undergoing IVF with GnRH antagonist for ovarian stimulation [29]. Furthermore, Dueholm’s review found an overall reduction in pregnancy rate with adenomyosis (RR 0.63) and an increased risk of miscarriage [20]. In contrast, a case-control retrospective study of 49 women with adenomyosis having oocyte donation showed no significant differences in implantation rates [30]. Benaglia’s [31] prospective cohort study also showed no significant difference in clinical or ongoing pregnancy rates in women with adenomyosis undergoing IVF. Similar findings have been found in other studies [32, 33].
Overall, the heterogeneity of studies investigating adenomyosis and infertility make it difficult to compare results. There are no high quality studies, with those published being limited by their retrospective nature, differing diagnostic criteria, small sample sizes, differing ages and concurrent endometriosis [7, 20, 34, 35]. There is also a lack of studies looking at the effect of adenomyosis on natural conception [20].
There are no current guidelines specific to the management of adenomyosis, especially for those seeking fertility assistance [36]. Medical treatment is limited in patients that wish to conceive and hysterectomy is not a valid option for these patients.[6] Growing desire for fertility preservation has seen an increase in cytoreductive surgeries performed and development of safer surgical techniques [37]. Whilst surgical excision of adenomyosis improves symptoms and fertility outcomes they are not without risk, including uterine adhesions and pregnancy complications such as uterine rupture [37, 38].
It is thought that GnRHa improves fertility in women with adenomyosis through reduction in hyper estrogenic states both indirectly through to hypothalamic-pituitary axis and directly at the level of the tissues by normalising the endometrial over-expression of aromatase cytochrome P450 that occurs in adenomyosis [39]. GnRHa has also been shown to have an antiproliferative and apoptotic effect on endometrial cells in vitro and Khan[40] identified suppressed pathologic lesions in women with adenomyosis. GnRHa therapy may improve endometrial receptivity by reducing the extent of basal endometrium dislocation that occurs in adenomyosis [41]. A retrospective cohort study by Bao et al[26] also found an increase in endometrial receptivity markers following long-acting GnRHa protocol and significant increase in clinical pregnancy rates in women with decreased ovarian reserve.
Over the last decade, four retrospective cohort studies have evaluated the use of GnRHa downregulation on fertility and IVF outcomes in adenomyosis[41-44]. Niu [41] compared clinical pregnancy rates in the first IVF cycle post treatment with GnRHa and HRT versus HRT alone. They found that one month of GnRHa downregulation doubled implantation and ongoing clinical pregnancy rates. Small case series further support the benefit of GnRHa downregulation prior to IVF [45, 46]. However following 2-3 months of monthly goserelin, Park [44] found no statistical difference in clinical pregnancy rates. A small RCT found no significant difference in outcomes[47]. Data regarding the effect of long term down regulation with GnRHa is limited [48].
We believe that adenomyosis can severely impair fertility and that our results further support this. Our cases were chosen for intervention following repeated failed IVF cycles, thought to be a result of their severe adenomyosis. Almost all of these patients had recurrent implantation failures prior to treatment with long term GnRHa downregulation. Our results show significant highly successful fertility outcomes post three months of GnRHa therapy prior to embryo transfer supporting its use in management of infertility. This differs from other evidence where successful cases with GnRHa downregulation were seen only following cytoreductive surgery [49-51]. Use of GnRHa therapy alone would likely be associated with less risks than in combination with surgical management, however whilst the women in our case series tolerated GnRHa therapy well (all successfully completing a minimum of three-months treatment), the side effects from a hypooestrogenic state may not be tolerated by all. Tolerance may be regime dependant and currently there remain large discrepancies in GnRHa downregulation regimes [41, 42, 44]. Our study has several limitations being retrospective in nature with a small sample size and high concurrent endometriosis rate. There was also selection bias as most patients had recurrent implantation failures in multiple cycles, this is also accentuated as the patients are their own controls. However, this significantly high success rate in a population that had very poor outcomes prior to this treatment is very promising. Acknowledging that outcomes will always be better after introduction of an intervention, we believe it supports further research to explore the impact of adenomyosis and GnRHa on implantation.
Conclusion
Our cases show promising results and support the use of long term GnRHa downregulation prior to frozen embryo transfer. The increased rate of pregnancy seen post GnRHa downregulation in this cohort may be attributed to increased endometrial receptivity promoted by GnRHa.
Furthermore, whilst we believe that GnRHa downregulation is a promising therapy for women with adenomyosis and infertility, further evidence is still required to assess its impact on pregnancy rates.
Sources / references
Table 1. Patient demographics
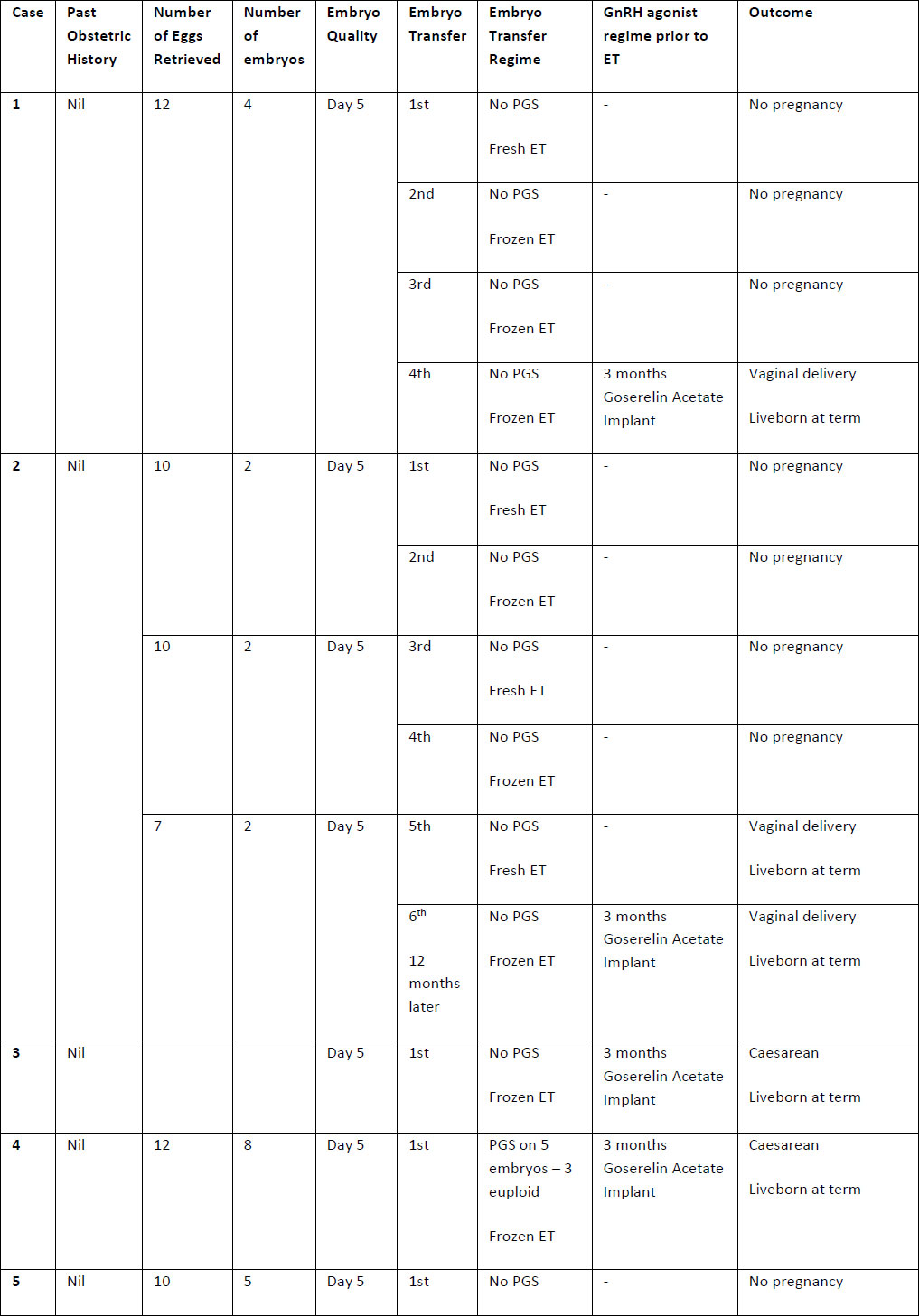
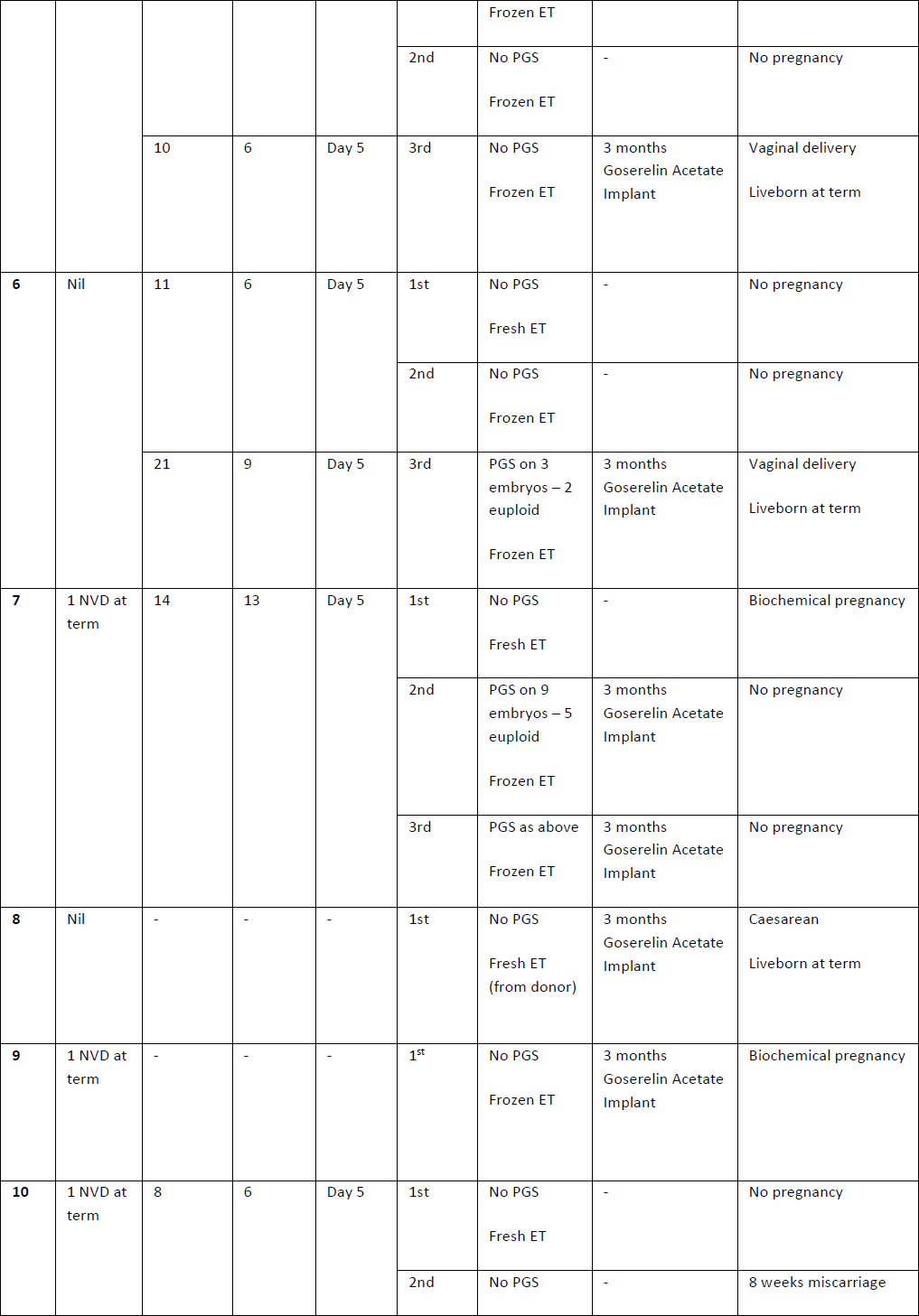
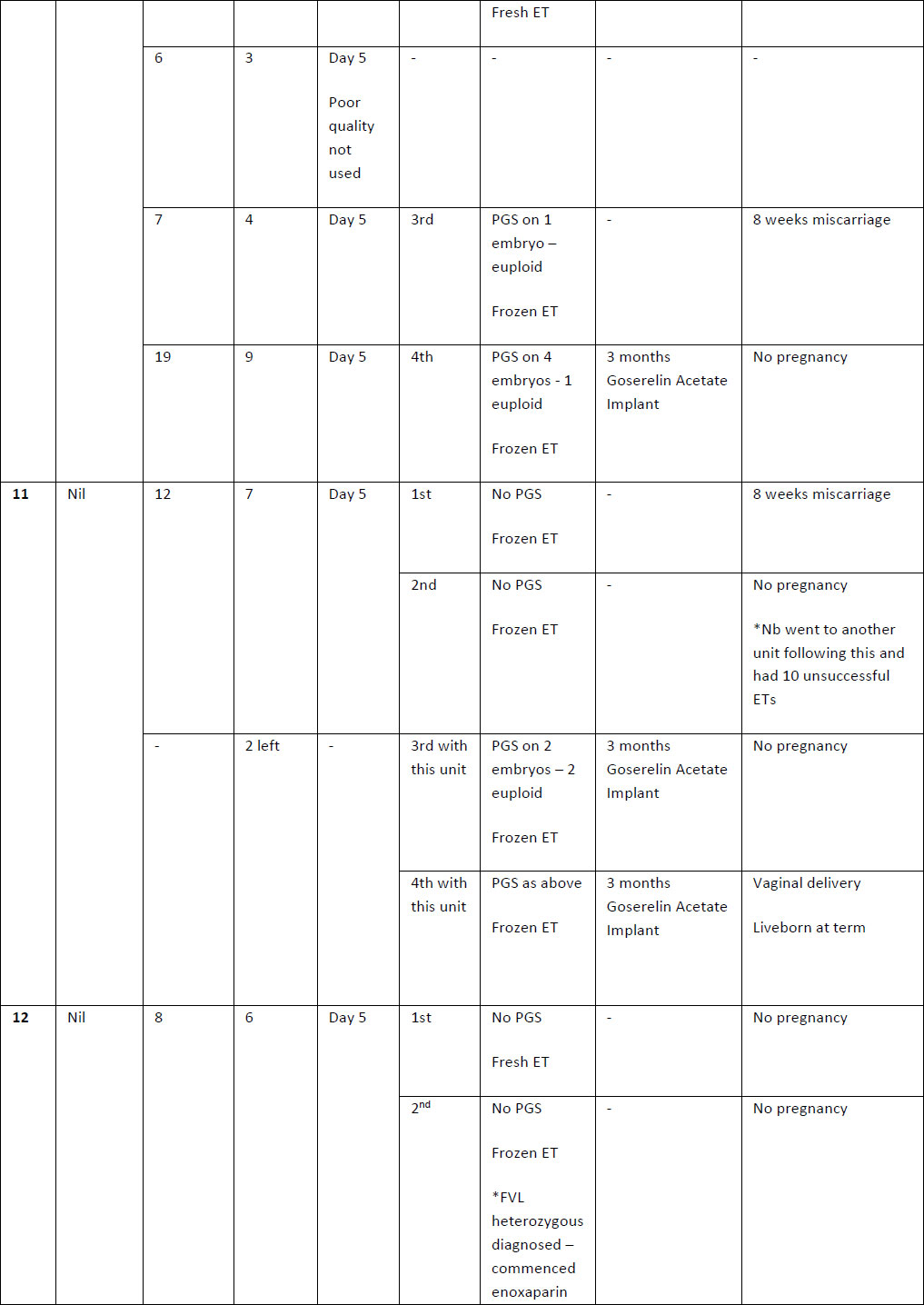
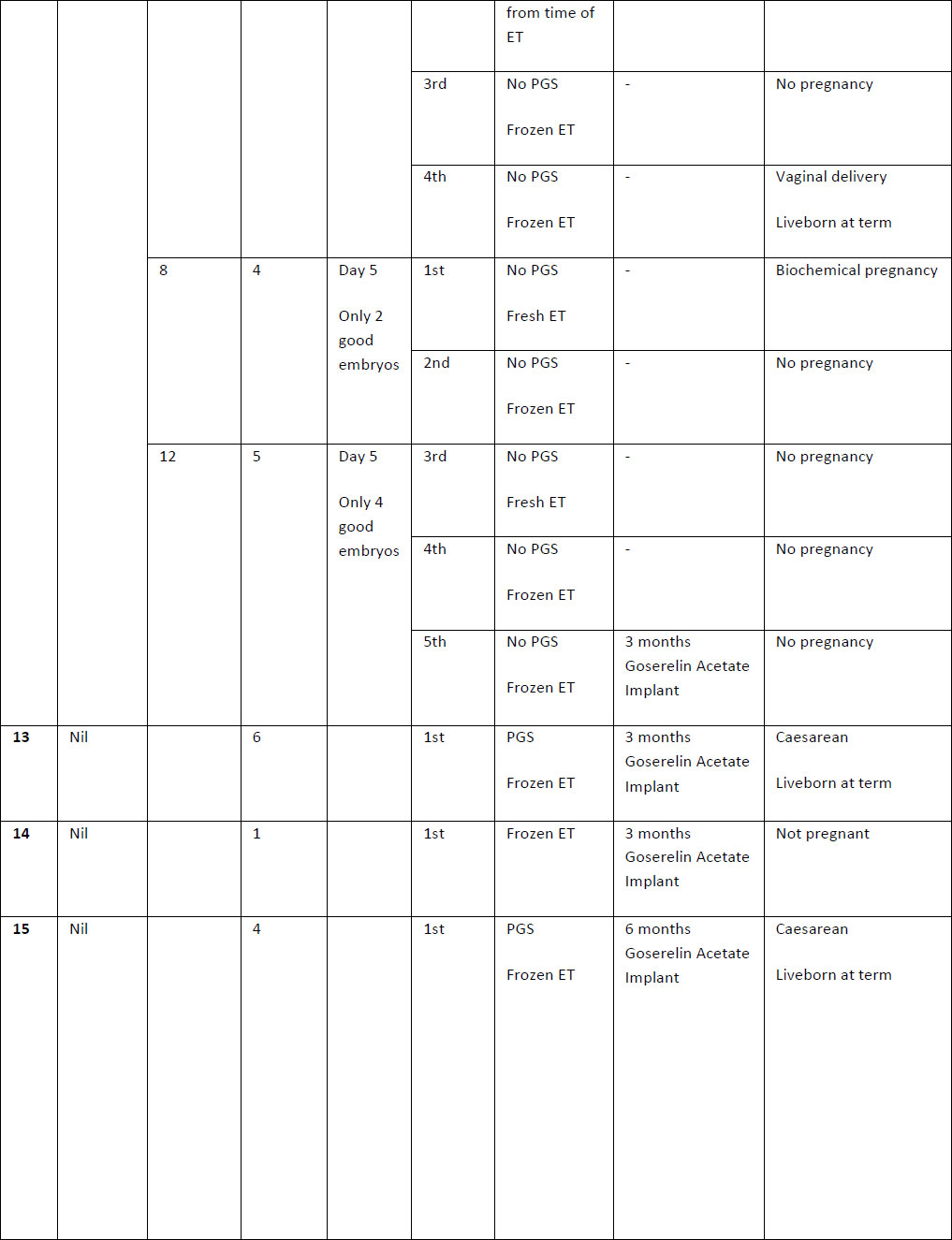
Table 2. Summary of case studies following GnRH agonist downregulation
The above table outlines the case series of seventeen pregnancies undergoing GnRH agonist down-regulation for adenomyosis prior to IVF.
Definitions: ET- endometrial thickness, Zoladex – goserelin acetate, PGS – Pre-implantation Genetic Screening
Figure 1: Suspected role of adenomyosis on fertility
The above figure outlines the key principles by which adenomyosis is thought to contribute to infertility. Adenomyotic lesions disrupt a proliferated junctional zone between the endometrium and myometrium, leading to uterine dysperistalsis and impaired sperm transport and blastocyst implantation. This is worsened by a hyperestrogenic state and reduced markers of endometrial receptivity that are critical to implantation.
LIF – leukaemia-inhibiting factor