Authors / metadata
DOI: 10.36205/trocar1.2020002
Introduction and context
As we look into history we see that pelvic floor reconstruction was built on native tissue repair since the introduction of anesthesiology in the middle of the 19th century until modern age [1]. Starting with pure shrinking, through tissue reduction and scar formation, strategies in the 19th century, like the Manchester approach or one step further the sacro-spinous fixation in the beginning of the 20th century. Ligaments were tied together or they were tightened as hard as possible. Besides the so called lateral defect repair (tightening of the lateral pelvic floor fascia) procedures like Mc Call sutures, Moszkowicz or culdoplasty were applied or are still in use[2-5].
From a scientific perspective these procedures are difficult to evaluate since there are no reliable data to be found as surgery was (is) generally authority based. In the sixties of the previous century Lane introduced the sacro pexy using an alloplastic transplant[6]. Due to this strategy a highly effective apical support was for the first time available. This approach still remains the „Gold Standard“in pelvic floor surgery although scientific proof is not very impressive. Randomized prospective studies are rare, essentially numerous single-center, low powered studies (N below 100) are available. Furthermore, long term follow-up data are infrequent and the pure volume (n=50 – 100 cases) of small single center studies define the term “Gold standard”.
In the late nineties of the previous century vaginal mesh-surgery was introduced and outplaced laparotomy but, in the process, did slow down the progress of the laparoscopic approaches. Due to the massive problems related to the vaginal mesh-surgery, a demand for alternative strategy is rising in Europe first followed by the rest of the world.
In England mesh is only allowed to be used under strict study conditions. New Zealand and Australia have nearly completely withdrawn the use of vaginal mesh. Numerus countries have regulated the use of mesh and lately the FDA forced the last mesh producing companies to withdraw their products from the market. Germany is one of the European countries without any regulation concerning mesh use but due to the latest EU directive for product security requesting study data for every single implant until 2021, things are bound to change. The diversity of implants will force the producers to reduce their port-folio as it will be impossible to provide data for every single product. Apart from that surgeons have to ask themselves, “shouldn´t we use as less mesh as possible?”. Surgeons ought to focus on risks for the patients due to the different materials and above all look at the success rates of a procedure putting emphasis on minimizing l side effects related to a specific surgical strategy.
What about native tissue repair ?
The German guidelines for pelvic floor repair formulated by the German Pelvic floor Society (AGUB) (http://www.awmf.org/uploads/tx_szleitlinien/015-006l_S2e_Descensus_genitalis-Diagnostik-Therapie_2016-11.pdf) analyzed 22 randomized studies regarding the anterior compartment. The variation in success rates is quite important and reaches 63% without and 67% with an additional apical fixation. In one long-term follow up 74% showed no new prolapse symptoms and only 7% underwent a second surgery varying in time from 6 -18 years during the follow up.
Regarding the posterior compartment, treated by colporrhaphy, the outcome is even better and cumulative success rates of 80% were reported (12 months follow up /19 studies). The few studies related to lateral defect repair quote good data but these studies scientific standards are low[7].
Multiple studies use POPQ classification to describe the defect and the measurement of success. POPQ is an optimal labeling metric change and describes with precision the anatomical correction. Unfortunately, POPQ is not linked to the clinical outcome concerning the individual patient. The International Urogynecology Journal (IUJ) reported that this has led to an undervaluation of multiple approaches in the last decades [8]. Already in 2011 the re-evaluation of a randomized trial comparing three different vaginal strategies was published in the American Journal of Gynecology and Obstetrics. The pure anatomical observation led to success rats 30-40%. After re-evaluation 8 years later the re-intervention rate was 1% and the clinical success rate related to satisfaction and wellbeing of the patients revealed close to 95%[9].
In summary it can be said that the traditional use of native tissue is underestimated. Unfortunately, the variation in surgical strategies does not allow to compare data. Multi-center studies are nonexistent and valid data i.e. dealing with dyspareunia or defecation disorders are rare. Certainly, in future the evaluation of clinical outcome should focus more on the clinical success rate and the risk profile of a specific surgical strategy.
What approaches are available ?
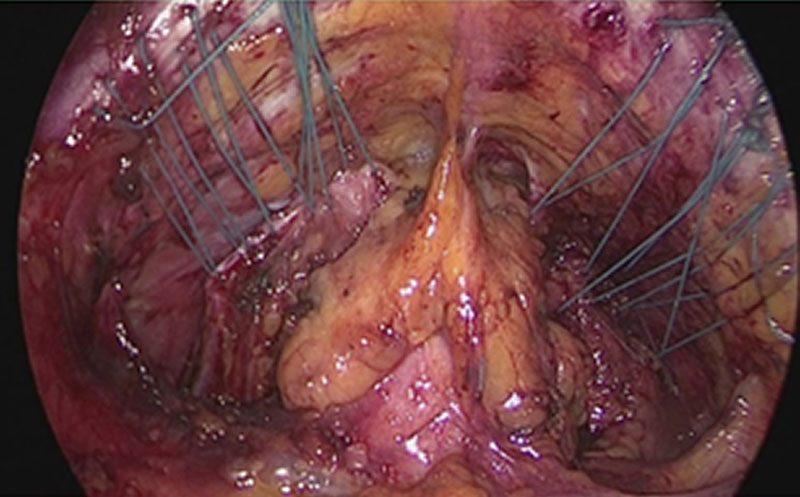
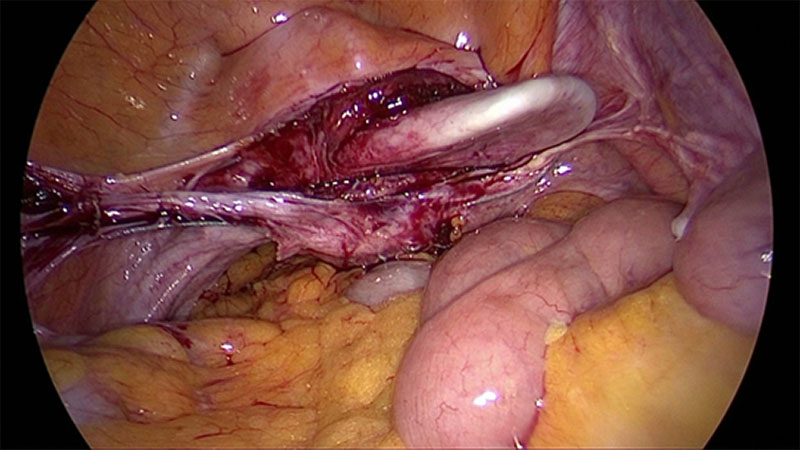
Sacro-colpopexy is widely used and often Y-shaped meshes are deeply positioned anteriorly and posteriorly to the vaginal wall for treating cystocele and rectocele in one and the same step. The opposite end is mainly fixed to the promontory, frequently by tackers. High success rates are reported but no convincing prospective multi-center trials are available [10-12]. De novo defecation disorders of 20% are tolerated as well as de novo Stress Urinary incontinence (SUI) of 10-50%. The use of Y-shaped meshes provides a high stability of the vagina, however the expanded tissue is not adjusted nor brought in its natural functional position. These tissues are often very thin and widened. Suturing a mesh on these tissues and then pull the mesh to be anchored in the sacrum can cause erosion by shear-force of pulling. Cystocele and rectocele are generally no longer clinically visible as these tend to disappear after sacro pexy through the pulling which leads to a dislocation of the apex cranially resulting an elongated vagina still thin and wide and a dislocation of the urethra. This maneuver can lead to a decreased pressure transmission to the urethra. This in turn can possibly explain the high de novo incontinence rates reported in the studies.
If the sacro pexy or the pectopexy technique is only used for apical support, this requires concomitant surgeries to cure defects in other compartments. Our group has called this a „Defect Oriented Strategy” (DOS). This way of working bares the risk of underestimating defects with as result secondary de novo defects. On the other hand, as the re-intervention rates are low and the intention to avoid over therapy, it is easy to explain and communicate this strategy well to the patients.
Data of our group (n=247) show that the combination of a laparoscopic sacro-colpo-pexy with vaginal colporrhaphy (anterior/posterior) and/or laparoscopic lateral defect repair lead to a cure rate over 90% In a follow up period of 24 months. The re-intervention rate was below 2%. [13]. As mentioned above a successful apical support seems to be the basis of a high cure rate. The same data could be measured in a randomized, prospective trial (sacro-pexy versus pecto-pexy) published in 2015 [14].
The success of native tissue repair depends on the biological tissue conditions. Finally, the “scaring” of the tissue has to be sustainable to provide the desired attempted form. Our group does not have valid parameters to measure these optimal outcome conditions yet. When the patient presents with a prolapse the duration of the clinical symptoms, the thickness and size of the defect and a relapse, after previous correction, are possible variables which can help to decide if an additional artificial material is necessary or not. Research materials, used in basic tissue research, are tested at this moment in time which probably in the future will support natural recovery. There are no clinically relevant results reported at this moment in time.
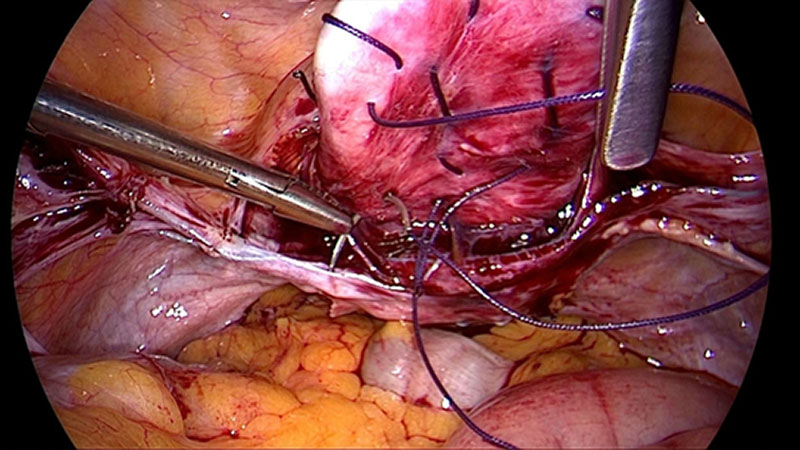
To provide a scare-less surgery for the vaginal mucosa our group has developed a laparoscopic approach for anterior and posterior midline defect and enterocele. The technique enables the surgeon to reduce length or width adapted to the size of the defect under direct vision. If the use of mesh seems to be necessary its use can be combined [15]. This approach generates the advantage that tissues close to the mesh material is augmented. This decreases the risk of erosion. Additionally as the vagina is not opened another risk factor for erosion (compared to vaginal approach) is eliminated. The adjustment of vaginal length and width by a special laparoscopic suture technique, results in a more natural position of the vagina and can reduce negative side-effects like urgency and de novo incontinence. However, the defect oriented strategy by combining multiple approaches is more sophisticated and time consuming.
Admittedly a sufficient apical support is carried out with support of mesh material. But the Defect Oriented Strategy (DOS) with targeted access to defect areas allows for a predominantly mesh-free surgery. Especially the laparoscopic approach allows the surgeon to approach all defects without generating scars in the vaginal wall. If the patient asks for a mesh-free strategy especially the young women with unclear desire for further children, a combination of native tissue techniques can be offered. Our group hopes to provide data in a very near future to formulate a clearer advice when a mesh is needed or when a native tissue strategy alone provides good long-term success.
In conclusion we can underline that native tissue repair builds an important base for pelvic floor repair. Laparoscopy provides for minimal tissue damage due to direct access to the defect. The use of artificial tissue in pelvic floor surgery should be considered very critically. To provide a comparable high quality in the long-term standardization of techniques multicenter studies a needed. The latter is also required to evaluate vaginal strategies.