Authors / metadata
DOI: 10.36205/trocar5.20240008
Abstract
This article explores how a gynaecological level 3 endovaginal ultrasound study can preoperatively determine the appropriate hysteroscopic surgical technique. It presents a treatment plan for a complex case involving an hypofertile patient with a distorted endometrial cavity due to multiple polyps and intramural leiomyomas. The use of the Bigatti shaver proved beneficial for delicately removing the polyps while preserving the remaining endometrium intact. However, considering the patient’s desire for pregnancy, the removal of an intramural fibroid protruding into the endometrial cavity was contemplated for a subsequent operation.
The preoperative and comprehensive gynaecological ultrasound study provided reassurance regarding which areas should be treated and offered information that modified the hysteroscopic approach to a twostep surgery. This approach aimed to reduce the risks associated with post-resection adhesions and intraoperative complications.
Conventional methods of polyp removal, such as mechanical removal or bipolar electric energy, while effective for treating symptoms like menorrhagia, do not significantly reduce the risk of intrauterine adhesion formation, which could directly impact the patient’s future ability to conceive. Polypectomy using hysteroscopic tissue removal devices, such as the Intrauterine Bigatti Shaver (IBS®), represents an advanced technique that minimizes the creation of adhesions. It should be considered as the primary instrument for use in hypofertile patients or those wishing to conceive postoperatively.
Case Discussion
A 39-year-old patient with a history of heavy periods experienced worsening menorrhagia over the past eight months. Examination and ultrasound revealed a distorted uterine cavity due to a combination of multiple fibroids and intrauterine polyps. The patient also presented with severe microcytic anemia (haemoglobin level of 7.5 mg/dL, mean corpuscular volume (MCV) of 60.3f, and ferritin < 4.0 ng/mL) due to iron deficiency, requiring urgent intravenous (IV) iron therapy using the Ferinject® protocol as a day-case hospital admission.
While waiting for the IV iron therapy to take effect on correcting the anemia, symptomatic treatment was initiated using nonsteroidal anti-inflammatory drugs (NSAIDs) and tranexamic acid, which effectively reduced bleeding and completely stopped it within seven days. Due to the persistence of symptoms, urgent hysteroscopic surgery was deemed necessary.
After 28 days following the IV iron infusion, the patient’s haemoglobin level normalized to 12.0 mg/dL, MCV increased to 74.8, and ferritin level rose to 54.3 ng/mL, allowing for the surgical procedure to proceed. The patient desires pregnancy and had cryopreserved five oocytes in the previous year. She has low anti-Müllerian hormone (AMH) levels and she is considered a low responder to ovarian stimulation.
The challenge in this case was to preserve the endometrial cavity as normal as possible to treat the menorrhagia effectively, avoid intraoperative complications, and prevent the formation of intrauterine adhesions, which could impede natural conception or successful embryo transfer in the future.
Preoperative ultrasound study
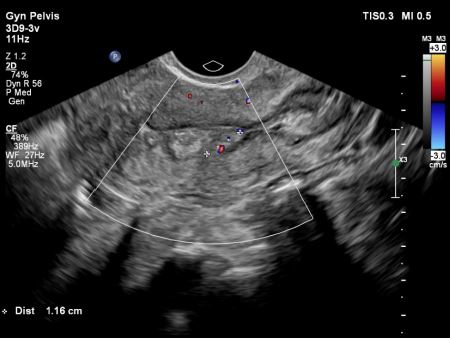
The patient has two fibroids: one subserosal fibroid located on the top left fundal part of the uterus, measuring 4-5 cm, and a second intramural fibroid situated just above the cervix’s isthmus on the left side, measuring 1.8 x 1.41 x 1.59 cm. The intramural fibroid is protruding indirectly into the endometrial cavity, as depicted in images 1 and 2.
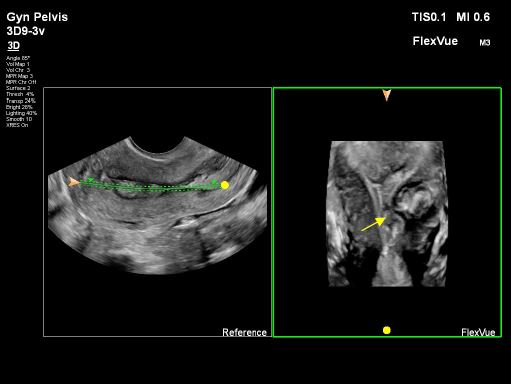
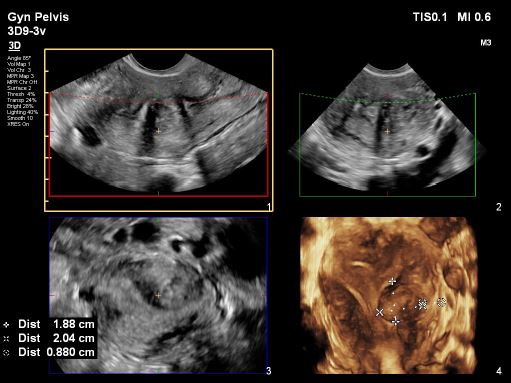
The fundal part of the uterus showed no septum but polyps were detected. These polyps, along with the thickened endometrium, were found in three areas: one in the upper right part near the ipsilateral tubal ostium (P1), and two others, one under the left side near the ostium (P2) and one just above the endometrium covering the intramural fibroid (P3), as depicted in images 3 and 4.All polyps had superficial contact withinthe middle part of the cavity, touching theendometrium covering the intramuralfibroid. Various imaging methods,including 2D, 3D plain mode, Flex view(ultrasound tomography mode), ColourDoppler, and Power Doppler, were usedto precisely assess the location,vascularity, and relationship with themyometrium features, as shown in images 5, 6, and 7. The serosa myometrium thickness covering the intramural fibroid measured between 0.8 and 0.7 cm.
Hysteroscopic surgery
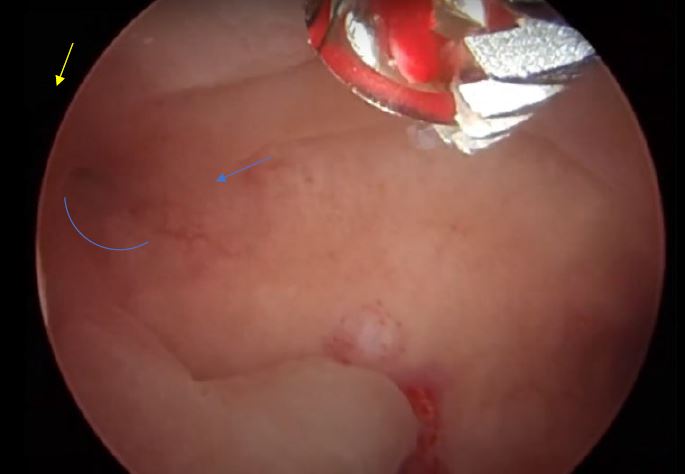
Following thorough informed consent, the patient underwent a day-case operative hysteroscopy using the Intrauterine Bigatti Shaver (IBS®). Initial images confirmed the preoperative ultrasound findings, showing all structures (thickened endometrium and polyps) in contact in the middle part of the cavity (images 8, 9, and 10). The polypectomy was successfully completed, as evidenced by images 11 and 12. The intramural fibroid caused minor modifications to the left lateral wall with thickened endometrium. Gentle shaving and “deroofing” were performed without penetrating deeply into the myometrium. Haemostasis was secured using a bipolar electrode after detecting the superficial vessel seen in the ultrasound (images 13, 14, and 15).
The patient underwent the operation during the second phase of her menstrual cycle. By the end of the procedure, the uterine cavity showed improved symmetry. Hyalobarrier® Gel was used as an additional measure to prevent the formation of intrauterine adhesions. The patient experienced a smooth recovery. The histology report confirmed the presence of submucosal segments of leiomyoma, segments of adenomatous endometrial polyp, and a section of endometrium in an advanced and partially abnormal secretory phase of the cycle. Postoperative follow-up included monthly ultrasound examinations for three months, which confirmed the maintenance of uterine cavity symmetry and the absence of polyp recurrence, as seen in the provided images. Further follow-up with endovaginal ultrasound studies and office hysteroscopy is scheduled in the near future. Recent ultrasound evaluation shows after three months postoperatively an improvement of the intrauterine cavity as shown in image 16. The patient, who is now in a new relationship, intends to attempt natural conception if possible. Long-term fertility assessment has been agreed upon.
Discussion
Ultrasound assessments are crucial for diagnosing medical conditions or preparing for surgery, ensuring procedures are performed under optimal conditions. However, the proficiency of gynecologists in ultrasound varies widely, both within and between European countries. The European Federation of Societies for Ultrasound in Medicine and Biology (EFSUMB) recognized this lack of standardization in training requirements for ultrasound practitioners across Europe and different medical specialties.
To address this issue, the EFSUMB introduced the “Minimum Training Requirements for the Practice of Medical Ultrasound in Europe” in 2002, with the latest guidelines specifically for gynaecological ultrasound training outlined in 2006 [1] . These guidelines establish three levels of minimum training and competencies for ultrasound practitioners, each accompanied by detailed syllabi and guidelines covering practical experience, theoretical knowledge, and technical skills assessment, see table 1.
Despite the existence of these guidelines since 2006, awareness among gynaecologists remains low, and few have completed all three training tiers. Level 3 competency is critical for achieving optimal imaging, precise measurements, and accurate preoperative assessments, including lesion characterization, margin delineation, volume determination, and assessment of vascularity. Utilizing multiple imaging modalities, such as 2D and 3D ultrasound, alongside vascular studies, supports therapeutic decision-making and facilitates informed patient consent by providing comprehensive information.
Failing to conduct a thorough preoperative ultrasound assessment may lead to unexpected complications during surgery or suboptimal surgical outcomes. Therefore, the selection of appropriate instruments and techniques relies heavily on the quality of preoperative ultrasound assessments. In cases where only basic 2D or 3D studies are conducted, there is a higher risk of encountering unforeseen challenges during surgery. Access to specialized instruments, like the Bigatti shaver, may also be limited, further emphasizing the importance of thorough preoperative planning and preparation.
Reduced fertility following hysteroscopic surgery can result from the development of intrauterine adhesions (IUAs) [ 2,3]. While hysteroscopic procedures effectively address various uterine issues, they can inadvertently harm the delicate endometrial lining, leading to adhesion formation within the uterine cavity. These adhesions disrupt the normal structure of the uterus, hindering implantation and affecting blood flow to the endometrium. Consequently, individuals may face challenges in conceiving or maintaining a pregnancy after surgery. The severity of fertility issues after hysteroscopic surgery depends on factors like the extent of adhesion formation and the individual’s reproductive goals. Managing reduced fertility often involves a multidisciplinary approach, including hysteroscopic assessment and adhesiolysis to remove adhesions, hormonal therapy to promote endometrial regeneration, and assisted reproductive techniques to enhance the chances of conception. In complex cases, especially in patients with known low ovarian reserve and complicated intrauterine anatomy, efforts should be made to minimize the risk of intrauterine adhesions as much as possible.
In the case example, the polyps were located in “mirroring locations,” and aggressive removal with a bipolar Transcervical Resection of Endometrium (TCRE) instrument could potentially lead to intrauterine adhesions, causing the endometrial surfaces of opposite sites to become stuck together over time. The thickened endometrium and polyp were positioned just under the ostium opening, making it challenging to remove the entire lesion without touching the ostium and causing thermal injury, even with a smaller caliber TCRE loop.
The Bigatti system offers advantages in this situation [4,5,6]. Its automated suction feature allows the lesion destined for removal to be enclosed within the probe, preventing mechanical injury to external adjacent tissues. Additionally, the cervical dilation required for introducing the Bigatti probe ( 19 French IBS ) is smaller compared to standard TCRE scopes. Standard resectoscopes vary between 26 and 24 French but nowadays we have 15 French resectoscope, reducing the risk of cervical trauma, which is particularly important for patients with fertility issues. The Bigatti system does not use thermal energy, minimizing thermal spread effects on the remaining endometrium and reducing the time needed to complete the polypectomy. Removal of polyp was necessary [7] to treat menorrhagia and irregular cycles but as well in order to restore a synchronized endometrium reducing local inflammation and thickness irregularities aiming to increase the chances for spontaneous conception or enhanced embryo transfer.
Regarding the intramural fibroid, the hysteroscopic view did not show significant intrauterine occupation. Thus, the decision was made to shave the endometrium above the intramural fibroid, between polyps P2 and P3. The appearance of the endometrium in this area was inflamed and altered. Hemostasis was ensured at the end of the operation.
Removing the superficial fibers of the intramural fibroid (deroofing) could facilitate future management if the fibroid continues to grow, potentially allowing it to protrude into the cavity and enabling Transcervical Resection of Fibroid (TCRF). However, postoperative follow-up is essential to assess anatomy stability, and complete or partial fibroid resection, combined with areas of endometrial polypectomy, may increase the risk of intrauterine adhesions due to a larger surface for re-epithelialization healing.
For this patient, achieving symmetry and proper reepithelialization of the endometrial cavity are critical for future pregnancy outcomes. Close monitoring and personalized treatment strategies are essential to address hypofertility effectively and improve reproductive outcomes for affected individual. These cases take time lot of effort from the patient and dedicated medical care.
Post-hysteroscopic resection intrauterine adhesions (IUAs) refer to the adhesions that may develop within the uterine cavity following a hysteroscopic surgical procedure. These adhesions, can result from trauma to the endometrial lining during the surgery.
The severity of IUAs can vary [8,9,10], ranging from mild adhesions that may cause minimal symptoms to severe cases leading to infertility or recurrent miscarriages. Management of post-hysteroscopic IUAs typically involves a combination of surgical intervention and adhesion prevention strategies [8,10].
Surgical techniques such as adhesiolysis are employed to remove the adhesions and restore the normal anatomy of the uterine cavity. Better results in regards fertility would be predicted if there is no creation of IUAs in comparison to patients who had IUAs of some degree and had to be treated with adhesiolysis.
There is a scarcity of articles demonstrating the importance of advanced imaging techniques preoperatively [11]. Recent guidelines from the International Society for Gynaecological Endoscopy (ISGE) emphasize the significance of thorough ultrasound assessment of the intrauterine cavity before any surgical intervention, including the removal of submucosal fibroids [12]. Similarly, close postoperative monitoring and long-term follow-up are crucial in managing post-hysteroscopic intrauterine adhesions (IUAs) to ensure optimal outcomes, especially for patients aiming to conceive. The challenge for hysteroscopists is to minimize the likelihood of such adhesion formation. Finally, the value of office hysteroscopy, when accessible to the patient, serves as an additional tool for objectively evaluating the intrauterine cavity, confirming desired endometrial healing, and detecting IUA recurrence.
Conclusion
The preoperative and complete gynaecological ultrasound study ideally of level 3 reassured which areas should be treated and provided information which modified the hysteroscopic approach to a 2-step surgery. If the patient were notconsidering future pregnancy, a more aggressive approach, involving fibroid resection, would have been pursued. However, in this instance appropriate gynaecological ultrasound advanced imaging study, the constrained external boundary of normal myometrium surrounding the fibroid, as well as the peripheral vascularity, posed significant challenges that warranted careful consideration, rendering the task notably demanding. The use of the Bigatti shaver was deemed less aggressive and less prone to causing adhesions compared to conventional resection techniques (TCRE) for polyps and / or fibroids. It is imperative to thoroughly explain our instruments and techniques to patients, allowing them to actively participate in therapeutic decision-making. This process necessitates comprehensive informed consent and an honest discussion regarding the limitations of the techniques, regardless of the expertise level of the hysteroscopist.
Acknowledgements
I extend my sincere thanks to Professor Bruno Van Herendael for his invaluable feedback and guidance throughout the development of this article.
References
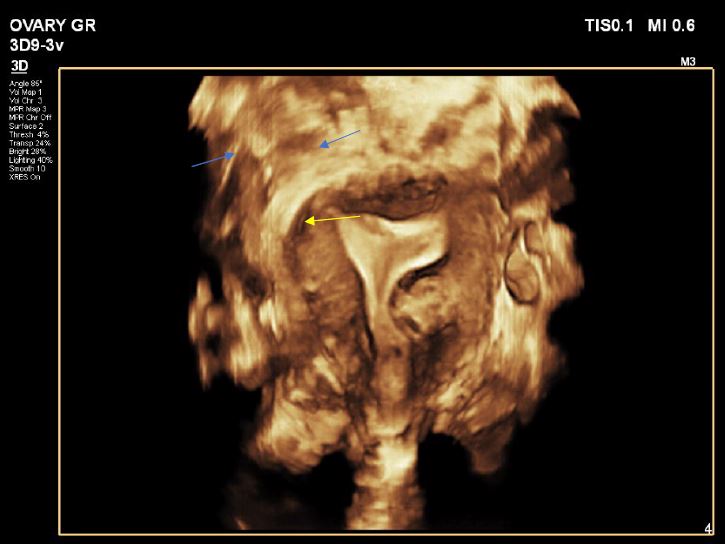
Image 1: 3D image in left rotation, showing the intramural fibroid yellow arrow, the fundal endometrial cavity is normal and the 3D slide show the ostium on both sides’ blue arrows
Image 2: Transverse measure / cut of the intramural fibroid note the peripheral vascularity towards the endometrium blue arrow, yellow arrow endometrium
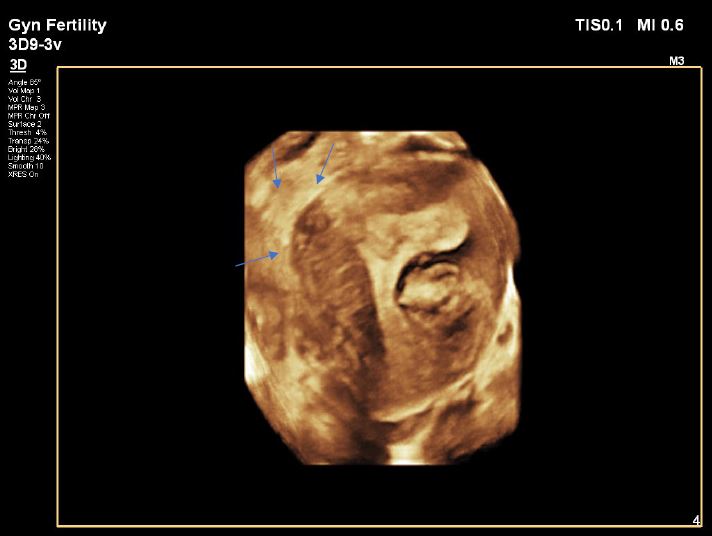
Image 3: Polyp locations on 3D mode, blue arrows: one under the right ostium presented as triangular longitudinal structure (P1), another one above the intramural fibroid (P2) a third one adjacent to top part of the isthmus from the left side (P3)
Image 4: Longitudinal view in midline, 2 parts of polyps, yellow arrow: polyp from the right( P1), and blue arrow shows polyp 3 ( P3). All polyps are in contact within the middle part of the endometrial cavity.
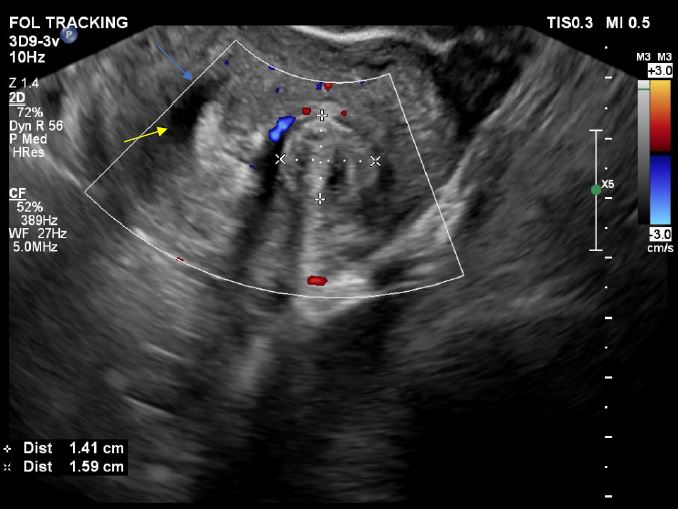
Image 5: Showing the vascular pedicle of the polyp (P3)
Image 6: Using ultrasound tomography mode / flexvue on Epiq Elite Phillips® machine. Selective slide from the 3D volume, is represented, and the P3 is detected with precision (yellow arrow), the 3D image is rotated to the right in order to see better the left ostium and cornual myometrium.
Image 7: 3D Study to assess the remaining (normal external) myometrium from the serosa assessing the operative risk and technical difficulty in case of left intramural myomectomy, the external limit was between 0.88- 0.7cm however the overall thickness of the left and right myometrium was not significantly different.
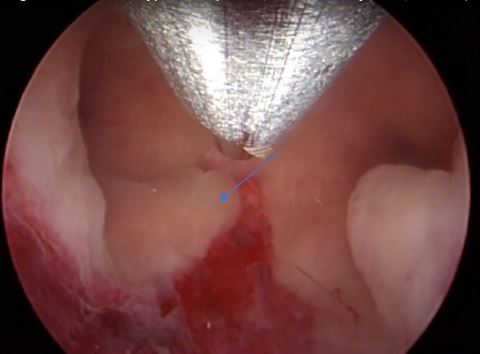
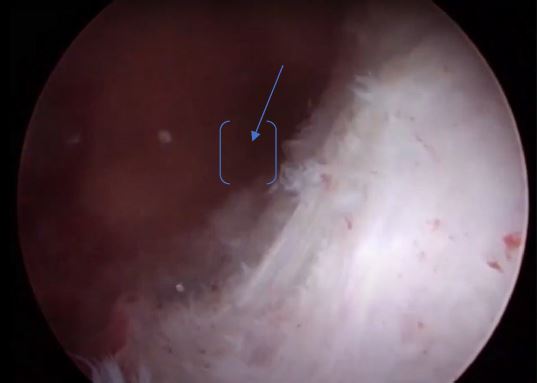
Image 8: Right ostium (yellow arrow), a longitudinal triangular polyp P1 (blue oblique line), on the top of the hysteroscopic image, the distal end of the Bigatti cutter. The blue arrow shows the imprint on the endometrium when the Bigatti blunt distal part touched the endometrial surface
Image 9: The fundal part of the cavity in this panoramic view, both ostia seen, note the inflamed endometrium under the right polyp (P1) at the middle part of the cavity, blue arrow, as well the polyp 2 (P2) from the left side.
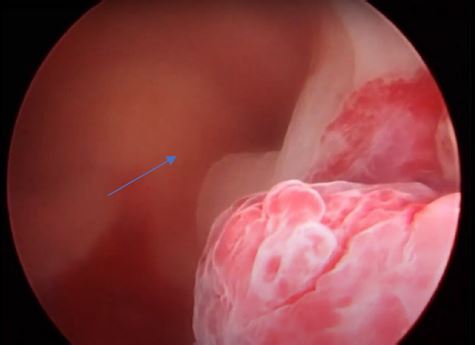
Image 10: Endometrial cavity, view of the left lateral part showing both left polyps, the P3 is the lowest and inflamed, behind is the P2 blue arrow.
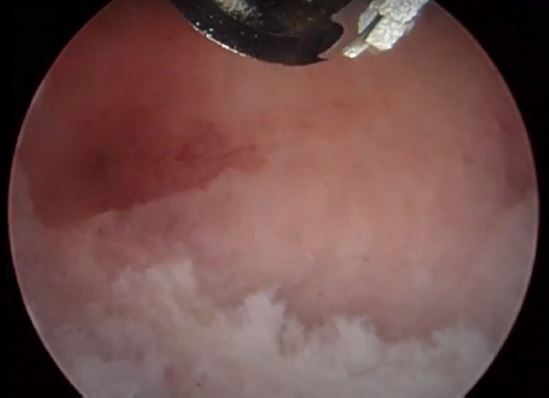
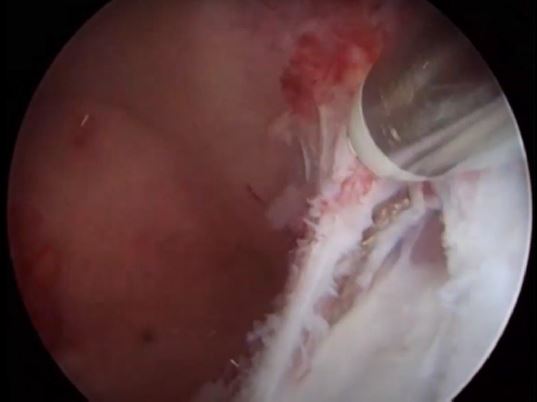
Image 11: Polypectomy completed on the right side (P1) the ostium is intact.
Image 12: Polypectomy was completed both ostia are clear and intact note that the fundal cavity is now normal in symmetry
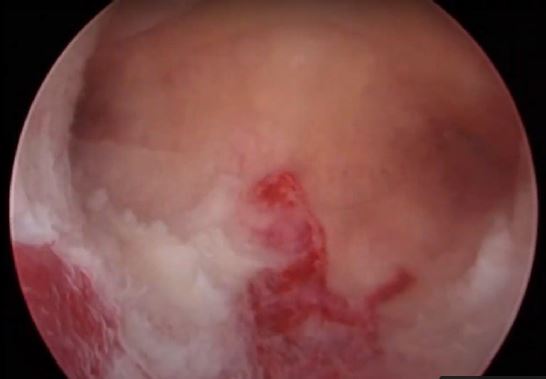
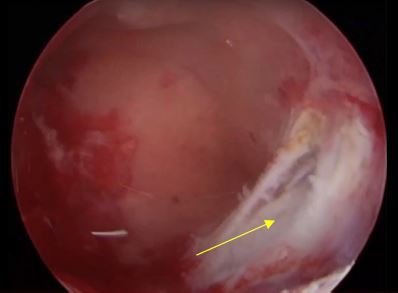
Image 13: After gentle shaving on the top part of the fibroid protuberance the area of the fibroid is seen blue arrow
Image 14: Haemostasis and coagulation of the superficial vessel feeding the intramural fibroid using Bigatti bipolar electrode
Image 15: Final result, note the deroofing imprint on the superficial part of the fibroid, yellow arrow
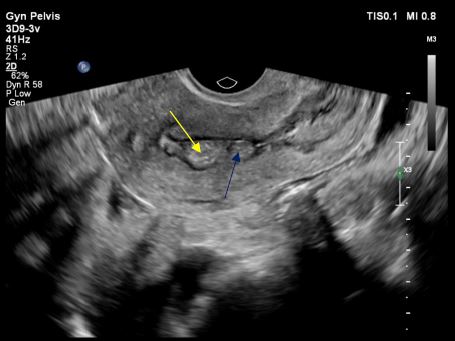
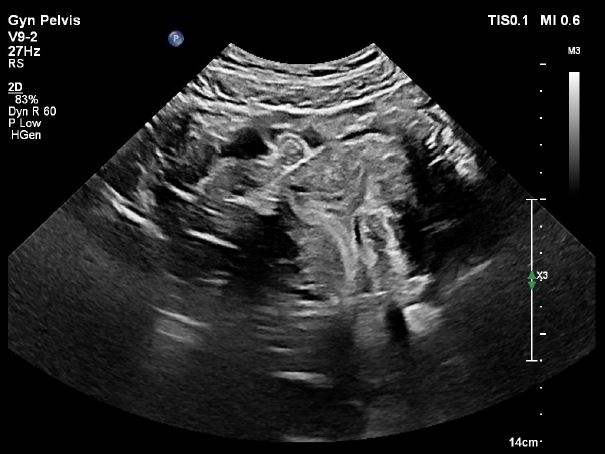
Image 16: Pelvic ultrasound image of the internal uterine cavity with symmetry above the cervical isthmus
Table 1: EFSUMB gynecological ultrasound competence levels